* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topic 2.2: Proteins
Rosetta@home wikipedia , lookup
Protein design wikipedia , lookup
Structural alignment wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein purification wikipedia , lookup
Protein moonlighting wikipedia , lookup
Homology modeling wikipedia , lookup
Protein domain wikipedia , lookup
Western blot wikipedia , lookup
Circular dichroism wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein folding wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
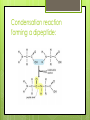
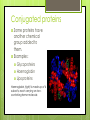
Topic 2.2a: Proteins Introduction CF is caused by a faulty channel protein in the cell surface membrane Water and salt transport is affected and mucus is too sticky. Protein Structure What monomer are proteins made up of? Amino acids How many different types of amino acids are there? 20 General stucture of amino acid: Identify the amino groups, carboxylic acid groups and R groups for each amino acid The amino acids Primary structure 2 amino acids can join together to form dipeptides. Condensation reaction: water lost. Many amino acids join to form a polypeptide. Condensation reaction forming a dipeptide: Primary Structure The sequence of amino acids in the polypeptide chain. The primary structure determines the tertiary structure and therefore the function. Secondary structure The C=O of the carboxyl group and the N-H of the amine group can be attracted to each other. Hydrogen bonds form between the H and the O. Alpha helix: H bonds form between every 4th amino acid Beta pleated sheets can also form: parallel chains joined by H bonds. Secondary structure The C=O of the carboxyl group and the N-H of the amine group can be attracted to each other. Hydrogen bonds form between the H and the O. Alpha helix: H bonds form between every 4th amino acid Beta pleated sheets can also form: parallel chains joined by H bonds. Tertiary structure Proteins have precise 3D shapes due to bending and folding of peptide chains. Caused by interactions between R groups. Covalent bonds (disulphide bridges) Ionic bonds Hydrogen bonds Also hydrophilic and hydrophobic interactions Tertiary structure Unique 3D folding of polypeptide due to interactions between the R groups. Types of interactions between R groups Covalent bonds (disulphide bridges) Ionic bonds Hydrogen bonds Hydrophillic and hydrophobic interactionshydrophobic R groups face inwards and the hydrophillic ones face outwards Tertiary structure The primary structure ensures that R groups are always in the same position, therefore the bonding between Rgroups will always be the same, and the hydrophobic and hydrophillic interactions will always be the same and therefore the tertialy structure of a specific protein is always identical. Image from Modis et al, Nature, 2004 Quaternary structure Proteins composed of more than one polypeptide chain have a quaternary structure Can be linked by H-bonds, ionic, or covalent bonds Example of quaternary structure: The influenza virus envelope protein Conjugated proteins Some proteins have another chemical group added to them. Examples: Glycoproteins Haemoglobin Lipoproteins Haemoglobin (right) is made up of 4 subunits, each carrying an ironcontaining heme molecule. Fibrous and Globular Proteins Use your textbook (p. 64) to complete the table comparing fibrous and globular proteins. Fibrous and Globular Proteins Use your textbook (p. 64) to complete the table comparing fibrous and globular proteins. Globular - haemoglobin Fibrous - Collagen Globular Proteins Irregular amino acid sequence Sequence highly specific and never varies between 2 examples of the same protein. Polypeptide chains folded into spherical shape. Length always identical in 2 examples of the the same protein. Relatively unstable structure. Soluble Metabolic functions Examples: all enzymes, some hormones (insulin), and haemoglobin Fibrous Proteins Repetitive regular sequences of amino acids- may vary slightly. Polypeptide chains form long parallel strands. Length of chain may vary in 2 examples of the same protein. Stable structure Insoluble Support and structural functions Examples: collagen and keratin Interactive Tutorial Activity 2.6