* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Pathways - PharmaStreet
Metabolic network modelling wikipedia , lookup
Metabolomics wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Peptide synthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Paracrine signalling wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biochemical cascade wikipedia , lookup
Butyric acid wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
Biochemistry wikipedia , lookup
A Biosynthetic Approach of
Medicinal Natural Products
Biosynthesis
• Formation of a chemical compound by a living organism.
• Biogenesis:
Production or generation of living organisms from other living organisms.
• Organisms vary widely in their capacity to synthesize and transform
chemicals. For instance, plants are very efficient at synthesizing organic
compounds via photosynthesis from inorganic materials found in the
environment, whilst other organisms such as animals and microorganisms
rely on obtaining their raw materials in their diet, e.g. by consuming plants.
• The pathways for generally modifying and synthesizing
carbohydrates, proteins, fats, and nucleic acids are
found to be essentially the same in all organisms,
apart from minor variations.
• These processes are collectively described as primary
metabolism, with the compounds involved in the
pathways being termed primary metabolites.
• Primary metabolism ( Biochemistry)
Secondary Metabolism
• Secondary metabolism, metabolic pathways that are
not essential for growth, development or reproduction,
but that usually have ecological function.
• Secondary metabolites are those chemical compounds in
organisms that are not directly involved in the normal
growth, development or reproduction of an organism. In
this sense they are "secondary".
• Secondary metabolites, are found in only specific
organisms, or groups of organisms, and are an
expression of the individuality of species.
• Secondary metabolites are not necessarily produced
under all conditions, and in the vast majority of cases
the function of these compounds and their benefit to
the organism is not yet known.
• Some secondary metabolites are produced for easily
appreciated reasons, e.g.
1. As toxic materials providing defense against
predators.
2. As volatile attractants towards the same or other
species.
3. As coloring agents to attract or warn other species.
• Secondary metabolism ( Natural products
chemistry).
The building blocks
• The building blocks for secondary metabolites are derived from primary
metabolism.
• The number of building blocks needed is surprisingly few.
•
The most important building blocks employed in the biosynthesis of
secondary metabolites are derived from:
1.
2.
3.
4.
5.
Acetyl coenzyme A (acetyl-CoA)
Shikimic acid
Mevalonic acid
1-deoxyxylulose 5-phosphate
Amino acids
1. Acetate pathway
• The form in which acetate is used in most of its
important biochemical reactions is acetyl
coenzyme A (acetyl-CoA).
• Acetyl-CoA is formed by oxidative decarboxylation
of the glycolytic pathway product pyruvic acid.
• Important secondary metabolites formed from
the acetate pathway includes:
1. Phenols
2. Prostaglandins
3. Macrolide antibiotics
2. Shikimate pathway
• Shikimic acid is produced from a combination of
phosphoenolpyruvate, a glycolytic pathway
intermediate, and erythrose 4-phosphate from the
pentose phosphate pathway.
• The shikimate pathway leads to a variety of:
1. Phenols
2. Cinnamic acid derivatives
3. Lignans
4. Alkaloids
3. Mevalonate pathway
• Mevalonic acid is itself formed from three molecules of acetyl-CoA, but the
mevalonate pathway channels acetate into a different series of compounds
than does the acetate pathway.
4. Deoxyxylulose phosphate pathway
• Deoxyxylulose phosphate arises from a combination of
two glycolytic pathway intermediates, namely pyruvic
acid and glyceraldehyde 3-phosphate.
• The mevalonate and deoxyxylulose phosphate
pathways are together responsible for the biosynthesis
of a vast array of terpenoid and steroid metabolites.
5. Amino acids pathway
• Peptides, proteins, alkaloids and many antibiotics are
derived from amino acids.
• Intermediates from the glycolytic pathway and the
Krebs cycle are used in constructing many of them.
• The aromatic amino acids phenylalanine, tyrosine,
and tryptophan are themselves products from the
shikimate pathway.
• Secondary metabolites can be synthesized by combining
several building blocks of the same type, or by using a
mixture of different building blocks.
• Many of secondary metabolites also contain one or
more sugar units in their structure.
• To appreciate how a natural product is elaborated, it is
of value to be able:
1. To dissect its structure into the basic building blocks
from which it is made up.
2. To propose how these are mechanistically joined
together.
What’s a phenolic compound?
A secondary product that contains a phenol group
- a hydroxyl functional group on an aromatic ring.
OH
Phenolics are a chemically diverse group: many
different properties and functions.
Biosynthesis of phenolics
Shikimic acid pathway is most common in plants. Converts
simple carbohydrates into aromatic amino acids. Not present
in animals.
Major types of phenolics
1. Simple phenolics - e.g. coumarins
2. Lignin - 2nd most abundant compound in plants
3. Flavonoids - two aromatic rings, 2 pathways
anthocyanins, flavones/flavonols
4. Condensed tannins
polymerized flavonoids
5. Hydrolyzable tannins
made of phenolic acids and sugars
smaller molecules than condensed tannins
Shikimic acid
Commonly known as its anionic form shikimate, is a cyclohexene, a
cyclitol and a cyclohexanecarboxylic acid.
It is an important biochemical metabolite in plants and microorganisms.
Its name comes from the Japanese flower shikimi the Japanese star
anise, Illicium anisatum), from which it was first isolated in 1885 by
Johan Fredrik Eykman.
The elucidation of its structure was made nearly 50 years later.
Shikimic acid is also the glycoside part of some hydrolysable tannins.
The shikimate pathway is a seven step metabolic route used by
bacteria, fungi, algae, parasites, and plants for the biosynthesis of
aromatic amino acids (phenylalanine, tyrosine, and tryptophan).
This pathway is not found in animals; therefore, phenylalanine and
tryptophan represent essential amino acids that must be obtained
from the animal's diet
Animals can synthesize tyrosine from phenylalanine, and therefore is not
an essential amino acid except for individuals unable to
hydroxylate phenylalanine to tyrosine).
Phosphoenolpyruvate and erythrose-4-phosphate react to form 2-keto3deoxy7phosphoglucoheptonic acid, in a reaction catalyzed by the enzyme DAHP
synthase.
2-keto3-deoxy7phosphoglucoheptonic acid is then transformed to 3-dehydroquinate
(DHQ), in a reaction catalyzed by DHQ synthase.
Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor,
the enzymic mechanism regenerates it, resulting in the net use of no NAD.
DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3dehydroquinate dehydratase, which is reduced to shikimic acid by the
enzyme shikimate dehydrogenase, which uses nicotinamide adenine
dinucleotide phosphate (NADPH) as a cofactor.
The next enzyme involved is shikimate kinase, an enzyme that catalyzes the
ATP-dependent phosphorylation of shikimate to form shikimate 3-phosphate.
Shikimate 3-phosphate is then coupled with phosphoenol pyruvate to give 5enolpyruvylshikimate-3-phosphate via the enzyme 5-
enolpyruvylshikimate-3-phosphate (EPSP) synthase.
Then 5-enolpyruvylshikimate-3-phosphate is transformed into chorismate by a
chorismate synthase.
Prephenic acid is then synthesized by a Claisen rearrangement of
chorismate by Chorismate mutase.
Prephenate is
oxidatively
decarboxylated with
retention of the hydroxyl
group by Prephenate
dehydrogenase to give
phydroxyphenylpyruvate
, which is transaminated
using glutamate as the
nitrogen source to give
tyrosine and αketoglutarate.
Role of Shikimic Acid Pathway:
• Starting Point in The Biosynthesis of Some Phenolics
Phenyl alanine and tyrosine are the precursors used in the biosynthesis of
phenylpropanoids. The phenylpropanoids are then used to produce the
flavonoids, coumarins, tannins and lignin.
• Gallic acid biosynthesis
Gallic acid is formed from 3-dehydroshikimate by the action of the enzyme
shikimate dehydrogenase to produce 3,5-didehydroshikimate. The latter
compound spontaneously rearranges to gallic acid.
Other compounds
Shikimic acid is a precursor for:
indole, indole derivatives and aromatic amino acid tryptophan and tryptophan
derivatives such as the psychedelic compound dimethyltryptamine.
many alkaloids and other aromatic metabolites.
Uses:
In the pharmaceutical industry, shikimic acid from the Chinese star anise (Illicium
verum) is used as a base material for production of oseltamivir (Tamiflu).
Target for drugs
Shikimate can be used to synthesize (6S)-6-Fluoroshikimic acid,
an antibiotic which inhibits the aromatic biosynthetic pathway.
Glyphosate, the active ingredient in the herbicide Roundup, kills
plants by interfering with the shikimate pathway in plants. More
specifically, glyphosate inhibits the enzyme 5enolpyruvylshikimate-3-phosphate synthase (EPSPS). "Roundup
Ready" genetically modified crops overcome that inhibition.
photosynthesis)
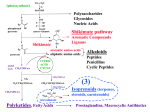
Polysaccharides
Glycosides
Nucleic Acids
phosphoenol
pyruvate
Shikimate pathway
Aromatic Compounds
Lignans
Shikimate
pyruvate
acetyl
CoA CH3COSCo
A
aromatic amino acids
aliphatic amino acids
Peptides
Penicillins
Cyclic Peptides
CITRIC
ACID
CYCLE
CH3COSCoA
-O
2CCH2COSCoA
Alkaloids
CH3COCH2COSCoA
(3)
CH3COSCoA
CH3COSCoA
Isoprenoids (terpenes,
steroids, carotenoids)
mevalonate
Prostaglandins, Macrocyclic Antibiotics
Polyketides, Fatty Acids
(3) Isoprene pathway: Terpenes
Terpenes, Steroids
Mevalonate
(3)
-- made from 5-carbon units
- C6 compound
that loses CO2
to form C5 units
phytane (C20)
a-pinene (C10)
cholesterol
(missing 3 C’s)
Isoprene Biosynthesis
decarboxylates
DMAP
to yield IPP
IPP
- Result: two isomeric 5-carbon molecules, IPP + DMAP
- Pyrophosphates: high-energy group powers biosynthetic rxns..
“nature’s leaving group”
acetate/mevalonate pathway
Biosynthesis of
terpenoids
Figure 24.7 The major subclasses of terpenoids are biosynthesized from the basic fivecarbon unit, IPP, and from the initial prenyl (allylic) diphosphate, dimethylallyl diphosphate,
which is formed by isomerization of IPP. In reactions catalyzed by prenyltransferases,
monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) are
Biosynthesis of Monoterpenes (C10)
DMAP
IPP
Prenyl transferase +
Geranyl pyrophosphate
(C10)
-OR-
Cyclase
enzymes
Farnesyl
pyrophosphate (C15)
rearranged to form
sesquiterpenes (C15)
cyclic monoterpenes
(C10)
Biosynthesis of Monoterpenes (C10)
(1) DMAP ionizes to form
electrophilic carbocation
(2) Nucleophilic attack by
IPP forms geranyl-PP
(3) Stereospecific loss of
HR, forming double bond
(4) Geranyl-PP ionizes,
rearranges to form a
carbocation intermediate
- Cyclic monoterpenes then form via enzyme-catalyzed
stereospecific rearrangements, functionalizations
thujone
Most cyclic monoterpenes have a distinctive odor
- basis of perfume & flavor industries
Stereoisomers have different characteristic smells
- demonstrates that smell receptors are 3D proteins,
i.e. chiral environments that can distinguish enantiomers
(+)-carvone = caraway seed
(+)-limonene = oranges
( )-carvone = spearmint
( )- limonene = lemons
-
-
Biosynthesis of Sesquiterpenes (C15)
DMAP
IPP
Prenyl transferase +
Farnesyl
pyrophosphate (C15)
rearranged to form
sesquiterpenes (C15)
Geranyl pyrophosphate
(C10)
Biosynthesis of Sesquiterpenes (C15)
DMAP
IPP
Geranyl pyrophosphate
(C10)
Prenyl transferase +
Farnesyl
pyrophosphate (C15)
rearranged to form
sesquiterpenes (C15)
- if you introduce a
labeled carbon in the
precursor, you can see
where it ends up in the
final natural product
Biosynthesis of Sesquiterpenes (C15)
DMAP
IPP
Geranyl pyrophosphate
(C10)
Prenyl transferase +
Farnesyl
pyrophosphate (C15)
rearranged to form
sesquiterpenes (C15)
- if you introduce a
labeled carbon in the
precursor, you can see
where it ends up in the
final natural product
Diterpene (C20) Biosynthesis
+
Farnesyl pyrophosphate
(C15)
carbocation
intermediate
Gerenylgerenyl-PP (C20)
Diterpenes
Gibberellin Biosynthesis
ound in most plants
CH3 oxidized, then lost as CO2
Gibberellin A3, a potent C19 plant hormone
(though originally isolated from a fungus)
Triterpene (C30) Biosynthesis
DMAP
IPP
Geranyl pyrophosphate
(C10)
Farnesyl pyrophosphate
(C15)
Squalene (from shark oil)
C30 compound: 2 farnesyl’s joined tail-to-tail
Triterpene (C30) Biosynthesis
Squalene
other C30 triterpenes
cholesterol (C27)
sex hormones
vitamin D
Cholesterol Biosynthesis
- lose 3 methyl
groups
Vitamin D Biosynthesis
- cholesterol from
liver is transported
to skin
- photochemically
converted into
vitamin D
- vitamin allows uptake
of essential calcium
Notes on Terpenes
(1) Oxidation reactions are carried out by the enzyme
cytochrome P450
- activate oxygen to introduce -OH, carboxyl groups
- allow removal of C’s through decarboxylation
(2) Triterpenes form flexible rings (chair, boat conformations)
with many chiral centers {rings usually not aromatic}
- provides a huge number of potential 3D structures
- high degree of biological activity
(3) Pathways can be elucidated using labeled precursors,
such as mevalonate with a 13C at position 2
- carbon NMR experiments reveal where the label ends
up in the completed molecule
Higher terpenes (C40)
2 x geranyl geranyl-PP
Lycopene
- major antioxidant pigment in tomatoes
b-carotene
- major accessory pigment in photosynthesis
The highest terpenes (Cbig #)
~ 1 % of plants can synthesize cis-polyisoprenoids, like rubber
H3C
Commercially used rubber plants can convert nearly 100% of
their mevalonate into rubber
photosynthesis)
Polysaccharides
Glycosides
Nucleic Acids
phosphoenol
pyruvate
Shikimate pathway
Aromatic Compounds
Lignans
Shikimate
pyruvate
acetyl
CoA CH3COSCo
A
aromatic amino acids
aliphatic amino acids
Alkaloids
Peptides
Penicillins
Cyclic Peptides
CITRIC
ACID
CYCLE
CH3COSCoA
-O
2CCH2COSCoA
CH3COCH2COSCoA
CH3COSCoA
CH3COSCoA
Isoprenoids (terpenes,
steroids, carotenoids)
mevalonate
andins, Macrocyclic Antibiotics
(4)
Polyketides, Fatty Acids
(4) Polyketide Biosynthesis
Polyketides
Acetate
(4)
O
H 3C
SCoA
Erythromycin A
(antibacterial)
Avermectin B1
(antihelminthic)
(4) Polyketide Biosynthesis
Polyketides:
(1) aromatic compounds, usually
with
O meta placement of -OH’s
(2) non-aromatic macrolides
H 3C
SCoA
6-methyl salicylate
Avermectin B1
(antihelminthic)
Acetate
(4)
Polyketide Biosynthesis
Compounds constructed by addition of successive 2-carbon units
Ketone may or may not be later reduced
Options in final structure:
OH
O
H
-or-
H 3C
C
H 3C
C
-or-
H 3C
C
Polyketide Biosynthesis
Important natural producers of polyketide metabolites:
- fungi (= molds)
- bacteria, particularly of the family Actinomycetes
- sea slugs
Polyketide Biosynthesis
Actual pathway starts with an acetyl-coA, but then successively
uses malonyl coA, which loses CO2 thus adding C2 units
- analogous to how mevalonate loses CO2 to add C5 units in
terpenoid biosynthesis
1 acetate, 3 malonyl coA’s
4 carbons are labeled
in the final product
The Lactone Mellein
[1,2-13C]-acetate
From the fungus Aspergillus
Growing polyketide chains are
held bound to the biosynthetic
enzyme, passed from one active
site to the next
Different active sites carry out the
various cyclizations + reductions
The final site is a thioesterase,
which cleaves the connection,
setting the compound loose
Mixed Biosynthesis: Vitamin K
isoprene unit
Synthesis performed by enteric bacteria in large intestine
Part of vitamin is from shikimate pathway, part isoprene
Rules for identifying pathways
(1) Is there nitrogen? Yes...alkaloid
(2) Count carbons
a) multiple of 5....terpene
C10= monoterpene
C15=sesquiterpene
C20 = diterpene
probably polyketide b) another even
number...
polypropionate
c) multiple of 3...
(w/ lots of methyls)
especially if the rings are
not aromatic