* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Kazzie`s Guide to Orgo 2
Enantioselective synthesis wikipedia , lookup
Discodermolide wikipedia , lookup
Bottromycin wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Hydroformylation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
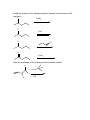
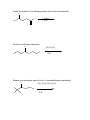
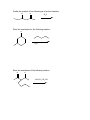
Guide to Orgo 2 General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify all the chiral centers in this molecule and label them all R/S: NH2 O Br H OH Show the mechanism of the following reaction: CH3I B:- Chapter 13 Draw the complete oxidation sequence of methane (hint, it starts with oxygen insertion to make methanol) into carbon dioxide and water. What are the reagents necessary for Swern Oxidation? What is the benefit of this reagent set? Show the mechanism for the following oxidation reaction (use a Bronstead acid base as necessary): O OH HO Cr OH O What color is Cr+6? Cr+3? What does adding Cr+6 to a substance determine? What is required to oxidize an aldehyde to a carboxylic acid? Predict the products of the following reactions; say what type of reaction they are and identify the functional groups involved: Cl CH3OH B:- NaH CH3CH2CH2OH CH3I Paying attention to stereochemistry, predict the following intramolecular reactions: OH I NaH OH I NaH Draw the reactive conformation chair form for the following intramolecular substitution reaction (initial stereochemistry not shown, show what it has to be) and predict the product: HO Br Show the following mechanism (draw out TsOH at least once in your mechanism) keeping track of the radioactive tracer: NaCl (a source of Cl-) TsOH CH3CH3CH218OH Including stereochemistry predict the product of the following epoxide ring opening reactions: O H3C NaOCH2CH2CH3 H HOCH2CH2CH3 Cl H3C Cl O H HOCH2CH2CH3 Cat. H2SO4 Why do we put in propanol with propanoxide in the top reaction as the solvent? Chapter 14 Predict the product of the following reactions: O Cl MgBr O OH Draw out the following mechanism: O CH3CH2SH H-B (cat.) Predict the product of the following reactions (assume an acid workup of all reactions): O NaBH4 H O NaH O C-Li H O LiAlH4 Draw the mechanism of the following imine formation reaction: O H2N H-B Predict the two organic products of the following reaction: O O H2O/H3O+ Predict all possible products from the following reaction (Hints: 1- There is one for the cis formation and one for the trans formation (therefore stereochemistry is important) 2- One never works with a single molecule 3-No acid is available): NaBH4 O Br Which is more reactive, formaldehyde or propanal? Why? What is the byproduct of all of the reversible nucleophile reactions in this chapter? How does this help increase the reversibility of these reactions? Chapter 15 Draw the reaction mechanism to form the acid chloride from the following starting material (picking the other reactant): O OH List three ways to make a carboxylic acid: Perform the following base condition mechanism: O NaOH/H2O Cl Perform the following acid condition mechanism and circle the important intermediate: O H3O+/H2O Cl Predict the product of the following reaction: O O + Cl O Show the mechanism of the following esterification reaction, and explain why the alcohol doesn’t attack directly. O OH Cl N This is a bit of an odd reaction, but think logically. Using your knowledge of Chem 210 and 215 predict the product of the following reaction: Ag2O O3 H3O+ NaOH Predict the product of the following reaction (hint: think intramolecular) O 1) NaBH4 2) H3O+ HO O Perform the following mechanism: O NH2CH2CH3 O H-B Because your professors seem to love it…a transesterification mechanism! O CH3CH2CH2CH2CH2OH O H-B Chapter 16 Find all 5 tautomers of the following compound: O O C C H H C H H H O H H C H Show the following enol formation mechanisms: H3O+/H2O O NaOH/H2O Predict the product of the following set of proton transfers: O O D2O Show the mechanism for the following reaction: O I H2O Show the mechanism of the following reaction: O O NaOCH3/CH3OH Br Chapter 17 Form an enol from the following molecule under acid conditions: O Use that enol with the following aldehyde and show the aldol condensation reaction (show the mechanism): O Show the following decarboxylation reaction (draw in the curved arrows): O OH O Predict the products of the following retroaldol reaction: O OH Bronstead Base Carbohydrates What is the generic structural formula for a carbohydrate? Draw the Fisher-projection of the simplest carbohydrate, name it. Then show the ACID catalyzed mechanism of it’s tautomerization to its ketose form and name that: What size ring is the kinetically favored hemi-acetal of a aldohexose? Thermodynamically favored? Show the ACID catalyzed mutorotation of the following molecule: N OH CH3 Draw the both products of an ACID catalyzed reaction of B-D Glucopyranose, circle the major product. Draw a sugar that if it was added to a solution of CuSO4 (which is blue/green) the solution would turn red and a precipitate would form. If you know it, what is that precipitate? Draw the product of the following reactions and predict the number of stereoisomers present in solution: HO HO O CH3CH2CH2Cl LiH OH OH HO HO O CH3CH2CH2OH HCl OH OH HO HO NH2 O HCl OH OH What is a glycoside? What is the result of the Tollins test for the following sugar? H3CO H3CO O H3CO OCH3 Draw an B(1->4) linkage of galactopyranose (the C-4 epimer of glucose) and glucopyranose (this is lactose): Draw an a(1->4) linkage of two glucopyranose molecules (this is maltose): What is poly-a-glucose? Poly-b-glucose? Amino Acids and Proteins NOTE: I am going to use any amino acid I want, if you don’t know what it looks like…look it up, it is good to get used to using anything they might throw at you. Draw the amino acid Gly at physiologic pH (~6.5), what is this form of a molecule called? Draw the titration curve for the amino acid Glu, draw the structure of the molecule at every important point: Predict the product of the following reaction: Lys + SOCl2 -> Draw the tripeptide GlnHisThr at pH 9: Circle all the amino acids in the following list with hydrophilic sidechains: Arg Pro Leu Asp Ile Val Glu Met Ser Gly Fill in the following blanks in the protecting group charts: O COCH3 NH3 O R COH NH3 O H R H COCH2Ph NH3 H R O COC NH3 H R O COCH3 NH3 H R O O COH COCH2Ph NH3 H3N H H R R O COC NH3 H R O O COH O O COH H3N N H H Ph R H R O O COH O N H H R O O COH O N H H O Ph R COH H3N O H R O COH O N H H R If given a choice of one of the following which is the most convenient to use and why? CBzPheAlaOtBu CBzPheAlaOMe BocPheAlaOtBu Draw the mechanism for removal the methyl ester protecting group from GlyOMe Draw DCC: What is the purpose of using DCC? Draw the mechanism for the reaction between CBzPhe and AlaOtBu using DCC to form CBzPheAlaOtBu: What is the direction of synthesis of a protein in a machine? In nature? What is the base material amino acids are connected to in solid state synthesis? What is the primary structure of a protein? Secondary? Tertiary? Quaternary? What is the most common kind of crosslinkage in protein structure? What amino acids is it between? What is the purpose of enzymes? DNA Draw the mechanism for the reaction between NAD+ and ethanol. What is the product? Which direction does a DNA chain grow in? How many H-bonds hold together Guanine and Cytosine? Which base appears in RNA but not DNA? Which carbon has it’s OH replaced by H in DNA? Draw the dinucleotide made up of thymine and adenine as it would appear in DNA: What is PG-Cl (draw it)? What is it used for in DNA synthesis? NOTE: Due to lack of notes on this section there is more you probably need to know, but I wanted to get this done in time for you to start it. Naming Name all of the following molecules using IUPAC naming. If marked with a “T” also give their common (trivial name) and IUPAC name. If a name is given, draw the molecule. Stereochemistry is important if implied by the name or structure. O OH Cl OH CH3 HO O O T: T: HOH2C CH2OH O OH O O OH OH HO O Br O OCH3 OCH3 OH Cl O O T: O HO OH O HC O T: O CH3 N CH3 (2R, 3R)-2,3,4 trihydroxybutanal (give trivial name also) Propanoyl butanoate Tetrahydrofuran Benzophenone 3-cyclohexeneone m-bromoacetophenone Diethylene glycol dimethyl ether (AKA: diglyme) 2,4-dimethyl-3-oxo-5-phenoxyhexanoic acid 2-hydroxypropanamide Isopentylbromide m,m-Diaminodiphenylmethane (I use this) n-hexylmagnesiumbromide p-toluene sulfonic acid Methanoic acid (also give trivial name) 2,4-dimethylcyclohexanecarboxylic acid Potassium benzoate ethyl propanoate 2,4-dihydroxyhexanoylchloride 3-methylhexanenitrile (9Z) Sodium octadec-9-enoate Good luck all and don’t hesitate to ask questions. E-mail: [email protected] Cell: (248)302-1975