* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Abl Protein-Tyrosine Kinase Inhibitor STI571 Inhibits In Vitro Signal
Cell growth wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Tissue engineering wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
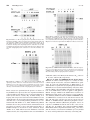
0022-3565/00/2951-0139$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Copyright © 2000 by The American Society for Pharmacology and Experimental Therapeutics JPET 295:139–145, 2000 Vol. 295, No. 1 2627/851335 Printed in U.S.A. Abl Protein-Tyrosine Kinase Inhibitor STI571 Inhibits In Vitro Signal Transduction Mediated by c-Kit and Platelet-Derived Growth Factor Receptors ELISABETH BUCHDUNGER, CATHERINE L. CIOFFI, NORMAN LAW,1 DAVID STOVER,2 SAYURI OHNO-JONES, BRIAN J. DRUKER, and NICHOLAS B. LYDON2 Novartis Pharma AG, Oncology Research, CH-4002 Basel, Switzerland (E.B., N.L., N.B.L.); Novartis Pharma, Summit, New Jersey (C.L.C.); and Division of Hematology and Medical Oncology, Oregon Health Sciences University, Portland, Oregon (S.O.-J., B.J.D.) Accepted for publication June 16, 2000 This paper is available online at http://www.jpet.org The protein-tyrosine kinase family can be divided into subgroups that have similar structural organization and sequence similarity within the kinase domain. The members of the type III group of receptor tyrosine kinases include the platelet-derived growth factor (PDGF) receptors (PDGF receptors ␣ and ), colony-stimulating factor (CSF)-1 receptor (CSF-1R, c-Fms), Flt-3, and stem cell or steel factor receptor (c-Kit). These receptor tyrosine kinases are characterized by five Ig-like domains in the extracellular domain and a cytoplasmic region containing a hydrophilic kinase insert domain (Heldin, 1995). PDGF receptors are normally found in connective tissue and glia but are lacking in most epithelia. However, recent studies have implicated paracrine or autocrine PDGF loops in the growth deregulation of gliomas and sarcomas as well as various human epithelial tumors. Furthermore, PDGF has been implicated in the pathogenesis of several nonmalignant proliferative diseases, including athReceived for publication February 21, 2000. 1 Present address: Prolifix Ltd., 91 Milton Park, Abington, Oxford Shire, OX 14 4RY, UK. 2 Present address: Kinetix Pharmaceuticals Inc., Suite 3500, 200 Boston Ave., Medford, MA 02155. of c-Met or nonreceptor tyrosine kinases such as Src and Jak-2 has been observed. In cell-based assays, STI571 selectively inhibited PDGF and stem cell factor-mediated cellular signaling, including ligand-stimulated receptor autophosphorylation, inositol phosphate formation, and mitogen-activated protein kinase activation and proliferation. These results expand the profile of STI571 and suggest that in addition to chronic myelogenous leukemia, STI571 may have clinical potential in the treatment of diseases that involve abnormal activation of c-Kit or PDGF receptor tyrosine kinases. erosclerosis, restenosis following vascular angioplasty (Ferns et al., 1991), and fibroproliferative disorders such as obliterative bronchiolitis (Hertz et al., 1992). c-kit is the cellular homolog of the v-kit retroviral oncogene. The c-kit gene product is expressed in hematopoietic progenitor cells, mast cells, germ cells, interstitial cell of cajal, and some human tumors (Nocka et al., 1989; Turner et al., 1992; Ishikawa et al., 1997). Studies with mice with inactivating mutations of c-kit or its ligand have demonstrated that the c-kit gene product is essential for maintenance of normal hematopoiesis, melanogenesis, gametogenesis, and growth and differentiation of mast cells and interstitial cell of cajal. In addition to its normal role, deregulation of c-Kit is thought to plan a role in certain human tumors, including germ cell tumors, mast cell tumors, gastrointestinal stomal tumors, small-cell lung cancers (SCLCs), melanoma, breast cancer, and neuroblastoma. In a number of tumors types, c-Kitmediated growth has been found to occur via mutation of c-kit, which results in ligand-independent activation of the receptor (Hirota et al., 1998; Longley et al., 1999; Tian et al., 1999). ABBREVIATIONS: PDGF, platelet-derived growth factor; SCLC, small-cell lung cancer; CML, chronic myelogenous leukemia; FCS, fetal calf serum; SCF, stem cell factor; IL, interleukin; FBS, fetal bovine serum; GM-CSF, granulocyte macrophage-colony-stimulating factor; DMEM, Dulbecco’s modified Eagle’s medium; MAP, mitogen-activated protein kinase; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2(4-sulfophenyl)-2H tetrazolium compound; Epo, erythropoietin. 139 Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 ABSTRACT STI571 (formerly known as CGP 57148B) is a protein-tyrosine kinase inhibitor that is currently in clinical trials for the treatment of chronic myelogenous leukemia. STI571 selectively inhibits the Abl and platelet-derived growth factor (PDGF) receptor tyrosine kinases in vitro and blocks cellular proliferation and tumor growth of Bcr-abl- or v-abl-expressing cells. We have further investigated the profile of STI571 against related receptor tyrosine kinases. STI571 was found to potently inhibit the kinase activity of the ␣- and -PDGF receptors and the receptor for stem cell factor, but not the closely related c-Fms, Flt-3, Kdr, Flt-1, and Tek tyrosine kinases. Additionally, no inhibition 140 Buchdunger et al. Materials and Methods CGP 57148 and STI571 (formerly known as CGP 57148B, the methane sulfonate salt of CGP 57148) were synthesized by Novartis Pharma AG (Basel, Switzerland). CGP 57148 is referred to as compound 1 in Zimmermann et al. (1997). No significant difference in results was seen between the two forms of the compound in in vitro studies, and both forms are referred to as STI571 in this study. For in vitro and cellular assays, a stock concentration of 10 mM STI571 was prepared in Me2SO4 and stored at ⫺20°C. Dilutions for all assays were freshly made before use. Liquid media, fetal calf serum (FCS), and media additives were from Life Technologies Inc. (Basel, Switzerland). PDGF-BB was from Life Technologies Inc., and PDGF-AA was from Bachem (Bubendorf, Switzerland). Stem cell factor (SCF) was from Genzyme (Cambridge, MA), Flt-3 was provided by Immunex (Seattle, WA), anti-c-Kit antibodies were from Oncogene Science (Cambridge, MA), anti-PDGF receptor ␣ antibodies were from Santa Cruz Biotechnology (sc338; Santa Cruz, CA), and anti-PDGF receptor ␣/ antibodies were from Upstate Biotechnology (06-495; Lake Placid, NY). Myo-[2-3H]inositol with PT6 polymer (10 –20 Ci/mmol) was from Amersham (Arlington Heights, IL), and the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation assay was from Promega (Madison, WI). Cell Lines. The 32D cell line is a murine myeloid cell line that is dependent on interleukin-3 (IL-3) for proliferation and was obtained from Joel Greenberger, University of Massachusetts Medical Center, Worcester, MA. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Upstate Biotechnology) and 15% WEHI-3B-conditioned medium as a source of IL-3. MO7e cells are a human megakaryocytic leukemia cell line that requires either granulocte macrophage-colony-stimulating factor (GM-CSF), IL-3, or stem cell factor (SCF) for proliferation and were obtained from G. Bagby, Oregon Health Sciences University (Portland, OR). Cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 2.5 ng/ml GM-CSF. Other cell lines that were used for these studies include 32D cells expressing v-src, courtesy of S. Anderson, Department of Pathology, State University of New York (Stony Brook, NY), and NIH 3T3 cells expressing c-fms (Varticovski et al., 1989). A10 rat aorta smooth muscle cells were obtained from the American Type Culture Collection (Manassas, VA) and were cultured in the suggested media and additives. M1 cells (American Type Culture Collection), a murine myeloid cell line that expresses Flt-3, were in grown in RPMI 1640 medium containing 10% FCS. Swiss 3T3 cells were kindly provided by G. Thomas (Friedrich-Miescher Institute, Basel, Switzerland). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS. In Vitro Kinase Assays. The kinase domains of Kdr, Flt-1, c-Met, and Tek were expressed in High Five cells (Invitrogen, San Diego, CA) using the Bac-to-Bac expression system (Life Technologies Inc.). The proteins were then purified to near homogeneity by standard chromatographic techniques. Kinase inhibition was measured by detecting the decrease in phosphorylation of poly(Glu, Tyr) essentially as previously described for the epidermal growth factor receptor (Buchdunger et al., 1994). The in vitro kinase assays were carried out under optimized assay conditions (ATP concentration ⬃Km), which allows a comparison of IC50 values for the different kinases. Jak-2 Kinase Assay. To determine whether Jak-2 was inhibited by STI571, 32Dp210 Bcr-Abl-expressing cells were serum starved for 8 h, and then stimulated for 10 min with 10% WEHI-3B-conditioned medium (IL-3 source). Nonidet P-40 lysates were prepared, and 500 g of lysate was immunoprecipitated with either anti-Jak-2 antibodies (5 l; Upstate Biotechnology), or anti-Abl antibodies (20 l K12; Santa Cruz Biotechnology), overnight at 4°C. Immunoprecipitates were bound to protein G Sepharose for 1 h, washed three times with PBS, and then with kinase buffer (20 mM Tris, pH 7.5, 10 mM MgCl2, 10 M sodium vanadate, 1 mM dithiothreitol). Kinase assays were performed with or without 10 M STI571, run on a 10% acrylamide gel, and exposed on a phosphorimager. Cell Extraction. Swiss 3T3 cells were grown to confluency in DMEM containing 10% FCS. Medium was replaced by DMEM containing 0.1% (w/v) BSA, and cells were incubated for 90 min with the indicated concentrations of STI571 before stimulation with PDGF-AA or PDGF-BB for 10 min. MO7e were serum starved (growth in serum-free medium for 16 h) and incubated for 90 min at 37°C with the drug before stimulation with recombinant human SCF (50 ng/ml) for 10 min at 37°C. M1 cells were washed twice with RPMI 1640 and resuspended at 2 ⫻ 106 cell/ml of RPMI containing 1% (w/v) BSA for 6 to 8 h. The indicated concentrations of inhibitor were added for the final 2 h of incubation. Cells were stimulated with 1 g/ml recombinant Flt-3 ligand (Immunex, Seattle, WA) for 5 min. c-Fms-expressing NIH 3T3 cells and v-Src-expressing 32D cells were incubated for 4 h in the presence of STI571 before lysis. To measure IL-3-stimulated tyrosine kinase activity, MO7e cells were washed twice with RPMI 1640 and resuspended at 2 ⫻ 106 cells/ml of RPMI 1640 containing 1% BSA. Cells were incubated in this medium for 6 to 8 h. Various concentrations of inhibitor were added for the final 4 h of incubation. At the end of this period, cells were stimulated with 10 ng/ml human recombinant IL-3 (gift of G. Bagby, Oregon Health Sciences University). Immunoprecipitation. c-Kit was immunoprecipitated from MO7e cell extracts containing 500 g of total protein using 1 g of anti-c-Kit antibody. Immunoprecipitation was carried out overnight at 4°C, and immunocomplexes were harvested by the addition of Pansorbin (Calbiochem, La Jolla, CA). After extensive washing, precipitates were resuspended in 40 l of 2⫻ concentrated SDS sample buffer. Similarly, PDGF receptors were immunoprecipitated from Swiss 3T3 cell extracts using anti-PDGF receptor ␣ or anti-PDGF receptor ␣/ antibodies. Western Blot Analysis. Equal amounts of protein from cell lysates were analyzed by Western blotting with anti-phosphotyrosine antibodies, anti-c-Kit antibodies, anti-PDGF receptor ␣ or antiPDGF receptor ␣/ antibodies. Bound antibodies were detected by using the ECL Western blotting system from Amersham (Amersham, UK). All experiments were performed at least in triplicate, and the effects of STI571 on cellular tyrosine phosphorylation were analyzed in a semiquantitative manner. Mitogen-Activated Kinase (MAP) Kinase Analysis. Serumstarved MO7e cells were incubated in the presence of the indicated concentrations of STI571 for 2 h at 37°C and then stimulated with 50 ng/ml human SCF for 5 min at 37°C. A10 smooth muscle cells were grown to 70% confluency in 6-well plates and starved in DMEM Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 Recently, we have described a protein-tyrosine kinase inhibitor (STI571/CGP 57148B) of the 2-phenylaminopyrimidine class that has selectivity for the Abl and PDGF receptor tyrosine kinases (Druker et al., 1996; Carroll et al., 1997; Zimmermann et al., 1997). In these reports, STI571 inhibited the Abl and PDGF receptor kinases at the in vitro enzyme, cellular, and in vivo levels. Based on its preclinical activity against Bcr-Abl, STI571 is currently in clinical trials for the therapy of chronic myelogenous leukemia (CML). In the current study, we have further extended the preclinical profile of STI571 against additional protein kinases from the type III group of receptor tyrosine kinases and other related receptor kinases. In addition to its previously reported inhibition of the Abl and PDGF receptor tyrosine kinases, STI571 was found to inhibit c-Kit, but did not affect closely related kinases such as c-Fms, Kdr, Flt-1, Tek, and Flt-3. The data also demonstrate that the compound is a potent inhibitor of c-Kit and PDGF-mediated signal transduction events in cells. In addition to CML, STI571 may have potential clinical utility in cancers and nonmalignant proliferative diseases involving deregulated c-Kit or PDGF receptor activation. Vol. 295 2000 Inhibition of Signal Transduction by STI571 (Fig. 1A). Pretreatment of cells with STI571 caused a concentration-dependent inhibition of PDGF-AA-stimulated PDGF receptor ␣-phosphorylation with an IC50 value of approximately 0.1 M (Fig. 1A). Tyrosine phosphorylation induced by the PDGF-BB homodimer was inhibited with a similar IC50 value as shown by Western blotting with total cell lysates (Fig. 1B, lanes 1–7) or PDGF receptor ␣-immunoprecipitated lysates (Fig. 1B, lanes 8 –14). These data indicate, by inference, that STI571 also inhibits PDGF receptor . The overall levels of expressed PDGF receptor protein did not change after exposure to STI571 (Fig. 1, A and B, bottom). STI571 was further tested for inhibition of type III receptor tyrosine kinases. Treatment of MO7e cells with SCF in the presence of STI571 resulted in a concentration-dependent inhibition of SCF-stimulated tyrosine phosphorylation with an IC50 value of approximately 0.1 M (Fig. 2A). Similar data were obtained by using anti-c-Kit immunoprecipitates followed by anti-phophotyrosine Western blotting (Fig. 2B). STI571 did not modulate the expression of c-Kit protein (Fig. 2B, bottom). In contrast, the compound did not affect the tyrosine phosphorylation pattern of c-Fms-expressing NIH 3T3 cells (Fig. 3) or Flt-3-stimulated tyrosine phosphorylation in myeloid M1 cells (Fig. 4) at concentrations up to 10 M. Because the tyrosine phosphorylation induced by c-Fms and Flt-3 ligand is dependent on c-Fms and Flt-3 kinase activity and STI571 did not change the pattern or intensity of tyrosine phosphorylation, this is consistent with a lack of inhibition of Fms and Flt-3 kinase activity by STI571. To extend the tests for specificity, various other protein-tyrosine kinases were assayed for inhibition of STI571. As shown in Fig. 5, no inhibition of tyrosine phosphorylation of v-Src nor any change in the overall phosphorylation pattern was seen by antiphosphotyrosine blotting with lysates of v-src-transfected myeloid 32D cells. IL-3 signal transduction is known to induce tyrosine kinase activity, including Jak-2. Therefore, 32D cells expressing Bcr-Abl were stimulated with IL-3 and lysed. Jak-2 and Abl were immunoprecipitated and in vitro Results Selectivity for Inhibition of Protein Kinases. STI571 was tested for inhibition of protein-tyrosine kinases of the PDGF receptor subfamily. Previous studies have shown that STI571 inhibits the PDGF receptor; however, these studies used ligand that activated both PDGF receptor ␣ and , so that is was not possible to determine whether STI571 inhibits each of these receptor subtypes. Because Swiss 3T3 cells possess significant numbers of both ␣- and -PDGF receptors (Kazlauskas et al., 1988), we investigated the effect of STI571 on the response of these cells to PDGF-AA and PGDF-BB. PDGF-AA binds to and activates only the PDGF receptor ␣, whereas PDGF-BB binds to and activates all PDGF receptors, including PDGF receptor ␣- and -homodimers and ␣-heterodimers (Heldin et al., 1988). PDGF-AA induced the tyrosine phosphorylation of a protein with a molecular mass of ⬃170 kDa, which was identified as the PDGF receptor ␣ by immunoprecipitation with anti-PDGF receptor ␣ antibodies Fig. 1. Inhibition of PDGF receptor autophosphorylation by STI571 in intact cells. Confluent Swiss 3T3 cells were incubated for 90 min with the indicated concentrations of STI571 before stimulation with PDGF-AA (20 ng/ml; A, top) or PDGF-BB (100 ng/ml; B) for 10 min. Whole-cell lysates were corrected for protein content and analyzed by Western blotting with anti-phosphotyrosine antibodies. In lanes 8 to 14, PDGF receptors were immunoprecipitated with anti-PDGF receptor ␣ antibodies (A) or antiPDGF receptor ␣/ antibodies (B). Bottom, lysates were analyzed by Western blotting with anti-PDGF receptor ␣ antibodies (A) or anti-PDGF receptor ␣/ antibodies (B). Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 containing 0.1% BSA for 18 h. After washing with PBS, cells were incubated in DMEM with the indicated concentrations of drug 2 h before stimulation with 20 ng/ml PDGF-BB for 5 min. After washing with ice-cold PBS containing vanadate, cells were lysed. Samples were analyzed by using the Phosphoplus MAP kinase antibody kit (New England Biolabs, Beverley, MA). In brief, samples were resolved on 10% SDS-polyacrylamide gel electrophoresis, and immunoblotted with phosphoMAP kinase antibodies. To control for equal loading of MAP kinase, samples were immunoblotted in parallel with anti-MAP kinase antibodies that recognize both phosphorylated and nonphosphorylated MAP kinase. Measurement of [3H]Inositol Phosphate Release. A10 cells were grown in 24-well plates (30,000 cells/well) and incubated with [3H]inositol (1 Ci/well) for 2 days in DMEM (without inositol) containing 10% FBS. On the day before the experiment, quiescence was induced by serum derivation for 24 h by replacing the medium with serum-free DMEM containing [3H]inositol (1 Ci/well). On the day of the experiment, quiescent cells were washed twice with Dulbecco’s phosphate-buffered salt solution and then incubated for 5 min with HEPES physiological salt solution (142 mM NaCl, 5.6 mM KCl, 2.2 mM CaCl2, 3.6 mM NaHCO3, 1.0 mM MgCl2, 5.6 mM D-glucose, 30 mM HEPES, pH 7.4) containing 0.1% BSA and 20 mM LiCl. Cells were preincubated with STI571 (50 l) for 30 min in a shaking (50 oscillations/min) water bath at 37°C. The reaction was initiated by the addition of 10 ng/ml PDGF (50 l) for a final volume of 500 l. After 5 min, the reaction was terminated by the addition of 0.2 ml HClO4 (10% v/v), and the plates were placed on ice for 15 min. The supernatants were transferred to glass test tubes, centrifuged (2000 rpm; 5 min), and neutralized with 1.5 M KOH containing 60 mM HEPES. [3H]Inositol phosphates were separated from the neutralized acid extracts by anion exchange chromatography. Measurement of Cell Growth. Cells were grown in 96-well culture plates (5000 cells/well) and allowed to adhere overnight. The cells were washed twice with Dulbecco’s phosphate-buffered salt solution, and quiescence was induced by replacing the medium 24 h before the start of the experiment with DMEM containing 0.2% FBS. Medium was removed from the wells and replaced with DMEM (without phenol red). Quiescent cells were incubated for 30 min with inhibitors and then stimulated with either 10 ng/ml PDGF or 10% FBS for 24 h. Cell growth was measured by using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation assay that measures the reduction, by living cells only, of the 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium compound (MTS). Briefly, 20 l of MTS is added to each well during the last 3 h of stimulation with mitogen. The absorbance at 490 nm was recorded by using a microplate reader. 141 142 Buchdunger et al. Fig. 4. Effects of STI571 on the Flt-3 ligand-stimulated tyrosine phosphorylaion. Serum-starved M1 cells were treated with the indicated concentrations of STI571 for 2 h. Equal amounts of cell lysate protein were analyzed by Western blotting with anti-phosphotyrosine antibodies. Migration of molecular weight markers (MW, Kd) is indicated on the left. Fig. 5. Effects of STI571 on the v-Src tyrosine kinase. 32D cells expressing v-src were incubated in the presence of the indicated concentrations of STI571 for 4 h. Equal amounts of cell lysate protein were analyzed by Western blotting with anti-phosphotyrosine antibodies. Fig. 3. Effects of STI571 on the c-Fms tyrosine kinase. NIH/3T3 cells expressing murine c-Fms were incubated with the indicated amount of inhibitor for 4 h before lysis. Lysates, normalized for protein content, were separated on 7.5% SDS-polyacrylamide gels, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine antibodies. kinase assays were performed in the presence or absence of 10 M STI571. As expected, there was significant inhibition of the Bcr-Abl tyrosine kinase, but no apparent inhibition of Jak-2 tyrosine kinase activity (Fig. 6). This finding is in keeping with our previous observations that STI571 was not antiproliferative in assays of IL-3-dependent growth of MO7e and 32D cells, or factor-independent proliferation of v-srctransformed 32D (Druker et al., 1996). Additionally, STI571 had little effect on the growth of normal committed blood cell precursors in response to IL-3 and erythropoietin (Epo) or G-CSF and Epo as measured in colony formation assays (Druker et al., 1996). When tested for inhibition of isolated enzymes in vitro, the compound showed no or weak inhibition of Kdr, Flt-1, Tek, and c-Met tyrosine kinases (IC50 values of 11, 25, 26.8, and 101 M, respectively). Effects on SCF- and PDGF-Mediated Signal Transduction. A common cellular response to a variety of extracellular signals involves the activation of MAP kinase pathways. To assay the phosphorylation of MAP kinases, an antibody that recognizes the tyrosyl phosphorylated forms of MAP kinases was used for immunoblotting cell lysates. Stimulation of MO7e cells with SCF resulted in the phosphorylation of two MAP kinases, known as pp44erk1 and pp42erk2. STI571 strongly inhibited the SCF-induced activation of MAP kinases with an IC50 value between 0.1 and 1 M (Fig. 7A). Similarly, STI571 treatment inhibited PDGF-BB-mediated MAP kinase activation in rat A10 smooth muscle cells (Fig. 7B). The same cell line was used to test the effect of STI571 on PDGF-mediated [3H]inositol phosphate release. The compound inhibited [3H]inositol phosphate release in response to PDGF-BB treatment with an IC50 value of 0.25 M (Fig. 8A). In addition, this compound inhibited PDGFBB-stimulated A10 cell proliferation with an IC50 value of 0.2 M. In contrast, concentrations of STI571 up to 10 M had no effect on A10 proliferation induced by serum (Fig. 8B). Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 Fig. 2. Inhibition of c-Kit autophosphorylation by STI571 in intact cells. Serum-starved MO7e cells were incubated for 90 min with the indicated concentrations of STI571 before stimulation with SCF (50 ng/ml) for 10 min. Whole-cell lysates were corrected for protein content and analyzed by Western blotting with anti-phosphotyrosine antibodies (A). B, c-Kit was immunoprecipitated with anti-c-Kit antibodies before Western analysis with anti-phosphotyrosine antibodies or anti-c-Kit antibodies (bottom). Vol. 295 2000 Inhibition of Signal Transduction by STI571 Fig. 7. Inhibition of MAP kinase phosphorylation by STI571. Serumstarved MO7e cells (A) or serum-starved A10 cells (B) were incubated with the indicated concentrations of STI571 2 h before stimulation with SCF (50 ng/ml; A) or PDGF-BB (20 ng/ml; B) for 5 min. Cells were lysed and analyzed by Western blotting with anti-phosphoerk antibodies. Discussion In this article we extended the in vitro profile of the protein-tyrosine kinase inhibitor STI571, which is currently in clinical trials for the treatment of CML. Previous studies have shown STI571 to be a selective inhibitor of Abl and PDGF receptor tyrosine kinases (Druker et al., 1996; Carroll et al., 1997; Zimmermann et al., 1997). However, these previous studies did not determine which of the PDGF receptor subtypes were inhibited by STI571. The current studies demonstrate that STI571 potently inhibits PDGF-AA-stimulated receptor phosphorylation. The finding that STI571 inhibits PDGF-AA-stimulated receptor phosphorylation in Swiss 3T3 cells indicates that the drug is a potent inhibitor of the PDGF receptor ␣. Because PDGF-BB homodimers bind and activate all possible PDGF receptor dimers (Heldin et al., 1988) and the PDGF-BB-stimulated receptor phosphorylation also was Fig. 8. Inhibition of PDGF-induced inositol phosphate formation and PDGF-induced proliferation of rat A10 smooth muscle cells. A, [3H]inositol-labeled A10 cells were preincubated with increasing concentrations of STI571 for 30 min and then challenged with 10 ng/ml PDGF for 5 min at 37°C. Basal [3H]inositol phosphate release remained unaffected by STI571. Values shown are the mean ⫾ S.E. obtained from three experiments. Where no error bar is shown, the error is within the size of the symbol. B, growth-arrested A10 cells were incubated with increasing concentrations of STI571 for 30 min before stimulation with 10 ng/ml PDGF or 10% serum at 37°C. The effects on proliferation were assessed with the colorimetric MTS reduction assay after 24 h of treatment. Values shown are the mean ⫾ S.E. obtained from three experiments. inhibited by STI571, we conclude that STI571 is a potent inhibitor of both PDGF receptor subtypes. In this manuscript, we demonstrate that STI571 also inhibits the c-Kit tyrosine kinase with essentially the same potency. Because c-Kit and PDGF receptors are members of the type III receptor tyrosine kinase family, other members of this family and closely related kinases were evaluated for inhibition by STI571. Interestingly, the related receptor tyrosine kinases Flt-3, Fms, Kdr, Flt-1, Tek, and c-Met were not inhibited. Because STI571 acts as an ATP-competitive inhibitor, this suggests that these tyrosine kinases, although structurally related, have subtle differences in the structure of their ATP-binding domains. These data extend our previous findings where STI571 has been shown to selectively inhibit the Abl tyrosine kinase in vitro (IC50 ⫽ 0.025 M) without affecting other tyrosine kinases such as epidermal growth factor receptor, c-Src, c-Fgr, c-Lyn, tyrosine protein kinase IIB, or serine threonine kinases such as protein kinase A, phosphorylase kinase, casein kinases 1 and 2, and Cdc-2/CycB (Druker et al., 1996). Similarly, a variety of other kinases involved in cytokine signaling (e.g., intracellular tyrosine kinases such as Src or Jak kinases) were not inhibited by STI571. The lack of inhibition of Jak-2 tyrosine kinase activity is in agreement with previously reported cellular results, demonstrating that STI571 lacks antiproliferation activity in IL-3-dependent proliferation and colony-forming assays (Druker et al., 1996). Activation of transmembrane tyrosine kinase receptors is generally associated with a variety of intracellular signaling events, including SH2- and SH3-mediated protein-protein interactions, and activation of signaling enzymes such as phospholipases C, protein kinase C, MAP kinases, and phosphatidylinositol 3-kinase. We have used the aorta vascular smooth muscle cell model to examine interference downstream of the PDGF receptor kinase. Although the exact contribution of these signaling pathways to vascular function remains to be elucidated, it has been demonstrated that activation of distinct signal transduction pathways contributes to the role of PDGF as a potent mito- Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 Fig. 6. Effects of STI571 on Jak-2 tyrosine kinase activity. 32D cells expressing Bcr-Abl were starved for 8 h. At the end of this period, cells were stimulated with 10% WEHI-3B-conditioned medium as a source of IL-3. Cells lysates were immunoprecipitated with Jak-2 (left two lanes) or Abl (right two lanes) antisera and in vitro kinase assays were performed in the presence (⫹) or absence (⫺) of 10 M STI571. The migration of Jak-2 and Bcr-Abl is indicated with an arrow. 143 144 Buchdunger et al. negative kinase-defective mutant of c-Kit into the SCLC cell line NCI-H209 markedly decreased the cells’ ability to grow in the absence of growth factors (Krystal et al., 1996), indicating the need for a c-Kit/SCF autocrine loop for growth. Furthermore, STI571 has been found to inhibit signal transduction and growth of SCLC cells (Krystal et al., 2000). Our findings further suggest that STI571 may have clinical activity in tumors and nonmalignant proliferative disorders with deregulated PDGF receptor and c-Kit signaling. Additionally, the encouraging results seen in clinical trials in CML may result from a combination of the activity of STI571 on Bcr-Abl and c-Kit on leukemia cells. Acknowledgments We thank M. Garay, P. Hauser, C. Koelbing, and V. Rigo for excellent technical assistance. We also thank S. Anderson for providing the 32D cell line expressing v-Src, G. Bagby for the MO7e cells and IL-3, and G. Thomas for the Swiss 3T3 cells. References Berdel WE, de Vos S, Maurer J, Oberberg D, von Marschall Z, Schroeder JK, Li J, Ludwig WD, Kreuser ED, Thiel E and Herrmann F (1992) Recombinant human stem cell factor stimulates growth of a human glioblastoma cell line expressing c-kit protooncogene. Cancer Res 52:3498 –3502. Buchdunger E, Trinks U, Mett H, Regenass U, Mueller M, Meyer T, McGlynn E, Pinna LA, Traxler P and Lydon NB (1994) 4,5-Dianilinophthalimide: A protein tyrosine kinase inhibitor with selectivity for the epidermal growth factor receptor signal transduction pathway and potent in vivo antitumor activity. Proc Natl Acad Sci USA 91:2334 –2338. Carroll M, Ohno Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, Gilliland DG and Druker BJ (1997) CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood 90:4947– 4952. Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J and Lydon NB (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2:561–566. Ferns GAA, Raines EW, Sprugel KH, Motani AS, Reidy MA and Ross R (1991) Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science (Wash DC) 253:1129 –1132. Forsberg K, Yalyi-Nagy I, Heldin CH, Herlyn M and Westermark B (1993) Plateletderived growth factor (PDGF) in oncogenesis: Development of vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad Sci USA 90:393–397. Gordon D (1992) Growth factors and cell proliferation in human transplant arteriosclerosis. J Heart Lung Transplant 11:S7. Heldin CH (1995) Dimerization of cell surface receptors in signal transduction. Cell 80:213–223. Heldin CH, Bäckström G, Oestman A, Hammacher A, Rönnstrand L, Rubin K, Nistér M, Westermark B, Backstrom G, Ostman A, Ronnstrand L and Nister M (1988) Binding of different dimeric forms of PDGF to human fibroblasts: Evidence for two separate receptor types. EMBO J 7:1387–1394. Hermanson M, Funa K, Hartman M, Claesson Welsh L, Heldin CH, Westermark B and Nistér M (1992) Platelet-derived growth factor and its receptors in human glioma tissue: Expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52:3213–3219. Hertz MI, Henke CA, Nakhleh RE, Harmon KR, Marinelli WA, Fox JM, Kubo SH, Shumway SJ, Bolman RM III and Bitterman PB (1992) Obliterative bronchiolitis after lung transplantation: A fibroproliferative disorder associated with plateletderived growth factor. Proc Natl Acad Sci USA 89:10385–10389. Hibi K, Takahashi T, Sekido Y, Ueda R, Hida T, Ariyoshi Y and Takagi H (1991) Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene 6:2291–2296. Hines SJ, Organ C, Kornstein MJ and Krystal GW (1995) Coexpression of the c-kit and stem cell factor genes in breast carcinomas. Cell Growth Differ 6:769 –779. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Shingo I, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM, Matsuzawa Y, Kanakura Y, Shinomura Y and Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (Wash DC) 279:577–580. Innui H, Kitami Y, Tani M, Kondo T and Ingami T (1994) Differences in signal transduction between platelet-derived growth factor (PDGF) A and B receptors in vascular smooth muscle cells. PDGF-BB is a potent mitogen, but PDGF-AA promotes only protein synthesis without activation of DNA synthesis. J Biol Chem 269:30546 –30552. Inoue M, Kyo S, Fujita M, Enomoto T and Kondoh G (1994) Coexpression of the c-kit receptor and the stem cell factor in gynecological tumors. Cancer Res 54:3049 – 3053. Ishikawa K, Komuro T, Hirota S and Kitamura Y (1997) Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: A comparison of control and Ws/Ws mutant rats. Cell Tissue Res 289:137–143. Kallio EA, Koskinen PK, Aavik E, Buchdunger E and Lemström KB (1999) Role of platelet-derived growth factor in obliterative bronchiolitis (chronic rejection) in the rat. Am J Respir Crit Care Med 160:1324 –1332. Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 gen and chemotactic factor for vascular smooth muscle cells (Innui et al., 1994). Moreover, PDGF has been implicated as an important factor involved in the vascular response to injury as observed in cardiovascular diseases such as atherosclerosis (Ross, 1995), restenosis (Pauletto et al., 1994), and transplant arteriosclerosis (Gordon, 1992). STI571 was shown to potently inhibit PDGF-induced inositol release, MAP kinase activation, and proliferation of A10 rat aorta smooth muscle cells. This finding suggests that STI571 may have therapeutic use in vascular disease states associated with smooth muscle cell proliferation and migration. In vivo studies also have recently confirmed the activity of STI571 or a structurally related compound (CGP 53716) in such systems (Kallio et al., 1999; Myllarniemi et al., 1999). Several studies have revealed that the majority of gliomas coexpress both PDGF and PDGF receptors, suggesting an autocrine mechanism of growth stimulation in this type of malignancy (Hermanson et al., 1992). High-grade primary gliomas express increased levels of PDGF receptors and their ligands compared with low-grade gliomas, indicating that in gliomas the presence of a PDGF autocrine loop correlates with tumor progression (Hermanson et al., 1992). There are several examples of malignant epithelial cells that produce PDGF but do not express PDGF receptors. Expression of PDGF in cultured malignant epithelial cell lines from human patients with breast (Perez et al., 1987) and prostate cancer (Sitaras et al., 1988) has been reported. Recent studies of plasma and tissue PDGF concentration in patients with breast cancer indicate that PDGF levels predict for shorter survival times and have an adverse effect on response to chemotherapy (Seymour et al., 1993). These findings suggest that tumor cell-derived PDGF also plays a role as a paracrine growth factor in tumorigenesis. Because PDGF is a potent mitogen and chemoattractant for both fibroblasts (Seppä et al., 1982) and endothelial cells (Smits et al., 1989), PDGF may have a function in tumor development by stimulating the growth of a supporting connective tissue stroma and contribute to endothelial cell growth. Development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB has been reported (Forsberg et al., 1993). Thus, STI571 also may have utility against such tumors that express PDGF receptors. SCF is thought to play a central role in the proliferation and differentiation of stem cells (McNiece and Briddell, 1995). Although SCF itself does not promote colony formation in vitro, it works in synergy with other growth factors such as GM-CSF, G-CSF, IL-3, IL-6, IL-7, and Epo to stimulate formation of both differentiated progenitor cells and more primitive multilineage progenitor cells of the myeloid and erythroid lineages (McNiece et al., 1991). In addition to its normal function, deregulation of SCF receptor signaling has been implicated in a number of human cancers, including SCLC (Hibi et al., 1991; Sekido et al., 1991; Krystal et al., 1996), breast carcinomas (Hines et al., 1995), glioblastoma (Berdel et al., 1992), testicular malignancies (Strohmeyer et al., 1991), gastrointestinal stromal tumors (Hirota et al., 1998), gynecological cancers (Inoue et al., 1994) and mastocytomas (Longley et al., 1999). Evidence for the involvement of inappropriate receptor signaling by c-Kit in SCLC comes from the finding that most SCLC cell lines and primary tumors overexpress SCF and c-Kit (Hibi et al., 1991; Sekido et al., 1991; Krystal et al., 1996). Introduction of a dominant- Vol. 295 2000 145 Takahashi T (1991) Preferential expression of c-kit protooncogene transcripts in small cell lung cancer. Cancer Res 51:2416 –2419. Seppä H, Grotendorst G, Seppä S, Schiffmann E and Martin GR (1982) Plateletderived growth factor is chemotactic for fibroblasts. J Cell Biol 92:584 –588. Seymour L, Dajee D and Bezwoda WR (1993) Tissue platelet derived-growth factor (PDGF) predicts for shortened survival and treatment failure in advanced breast cancer. Breast Cancer Res Treat 26:247–252. Sitaras NM, Sariban E, Bravo M, Pantazis P and Antoniades HN (1988) Constitutive production of platelet-derived growth factor-like proteins by human prostate carcinoma cell lines. Cancer Res 48:1930 –1935. Smits A, Hermansson M, Nister M, Karnushina I, Heldin CH, Westermark B and Funa K (1989) Rat brain capillary endothelial cells express functional PDGF B-type receptors. Growth Factors 2:1– 8. Strohmeyer T, Peter S, Hartmann M, Munemitsu S, Ackermann R, Ullrich A and Slamon DJ (1991) Expression of the hst-1 and c-kit protooncogenes in human testicular germ cell tumors. Cancer Res 51:1811–1816. Tian Q, Frierson HF Jr, Krystal GW and Moskaluk CA (1999) Activating c-kit gene mutations in human germ cell tumors. Am J Pathol 154:1643–1647. Turner AM, Zsebo KM, Martin F, Jacobsen FW, Bennett LG and Broudy VC (1992) Nonhematopoietic tumor cell lines express stem cell factor and display c-kit receptors. Blood 80:374 –381. Varticovski L, Druker B, Morrison D, Cantley L and Roberts T (1989) The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature (Lond) 342:699 –702. Zimmermann J, Buchdunger E, Mett H, Meyer T and Lydon NB (1997) Potent and selective inhibitors of the Abl kinase - Phenylamino-pyrimidine (PAP) derivatives. Bioorg Med Chem Lett 7:187–192. Send reprint requests to: Dr. E. Buchdunger, Novartis Pharma AG, Oncology Research, K-125.416, CH-4002 Basel, Switzerland. E-mail: elisabeth. [email protected] Downloaded from jpet.aspetjournals.org at ASPET Journals on August 3, 2017 Kazlauskas A, Bowen Pope D, Seifert R, Hart CE and Cooper JA (1988) Different effects of homo- and heterodimers of platelet-derived growth factor A and B chains on human and mouse fibroblasts. EMBO J 7:3727–3735. Krystal GW, Hines SJ and Organ CP (1996) Autocrine growth of small cell lung cancer mediated by coexpression of c-kit and stem cell factor. Cancer Res 56:370 – 376. Krystal GW, Honsawek S, Litz L and Buchdunger E (2000) The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res, in press. Longley BJ Jr, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu SZ, Heitjan D and Ma Y (1999) Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA 96:1609 – 1614. McNiece IK and Briddell RA (1995) Stem cell factor. J Leukocyte Biol 58:14 –22. McNiece IK, Langley KE and Zsebo KM (1991) Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol 19:226 –231. Myllarniemi M, Frosen J, Calderon Ramirez LG, Buchdunger E, Lemstrom K and Hayry P (1999) Selective tyrosine kinase inhibitor for the platelet-derived growth factor receptor in vitro inhibits smooth muscle cell proliferation after reinjury of arterial intima in vivo. Cardiovasc Drugs Ther 13:159 –168. Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A and Besmer P (1989) Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—Evidence for an impaired c-kit kinase in mutant mice. Genes Dev 3:816 – 826. Pauletto P, Sartore S and Pessina AC (1994) Smooth muscle cell proliferation and differentiation in neointima formation and vascular restenosis. Clin Sci 87:467– 479. Perez R, Betsholtz C, Westermark B and Heldin CH (1987) Frequent expression of growth factors of mesenchymal cells in human mammary carcinoma cell lines. Cancer Res 47:3425–3429. Ross R (1995) Growth regulatory mechanisms and formation of the lesions of atherosclerosis. Ann NY Acad Sci 748:1– 4. Sekido Y, Obata Y, Ueda R, Hida T, Suyama M, Shimokata K, Ariyoshi Y and Inhibition of Signal Transduction by STI571