* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Universal Fusion/Expression Profile
Genome (book) wikipedia , lookup
Microevolution wikipedia , lookup
Gene therapy wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Public health genomics wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Oncogenomics wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene expression profiling wikipedia , lookup
Nutriepigenomics wikipedia , lookup
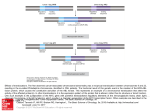
White Paper Comprehensive Detection of Known/Novel Fusions to Aid in the Diagnosis of Hematologic Disorders, Soft Tissue Neoplasms, and Solid Tumor Diseases Using the Universal Fusion/Expression Profile Lawrence M. Weiss, MD, Medical Director. NeoGenomics Laboratories, Aliso Viejo, California Universal Fusion/Expression Profile Expanding the Boundaries of Cancer Diagnostics Numerous gene translocations are associated with specific neoplasms. Currently, testing for translocations is routinely performed using platforms such as FISH or a molecular test that is designed to detect a small subset of translocations. Although these methodologies are proven to be very powerful tools for identifying translocations, there are instances where an alternative approach is necessary due to factors such as limited sample availability or lack of access to a clinically validated test that can target specific translocations. In order to provide researchers and clinicians with an alternative testing modality that can be utilized in various scenarios, NeoGenomics Laboratories is now offering the Universal Fusion/Expression Profile. This assay is one of the first clinically validated next-generation sequencing based profiles that is capable of detecting fusion transcripts and analyzing gene expression in 1,385 genes with particular implications in solid tumors, soft tissue cancers, and hematologic diseases. This test covers chromosomal translocations that are not currently covered by FISH and cases where a specific molecular translocation test is not available. Translocations that lead to deregulation of expression can be addressed by this test when compared to the expression of a proper normal control. The Universal Fusion/Expression Profile sequences all exon regions of the 1,385 cancer genes included as part of this profile and has been validated on bone marrow, peripheral blood, FFPE and fresh tissue. The RNA-based approach allows for the efficient and highly sensitive identification of thousands of gene fusions and unusual variants, all in one analysis. Hematologic disorders, soft tissue neoplasms, and rare solid tumors are most often associated with specific translocations. These neoplasms range from the common BCR-ABL1 associated chronic myeloid leukemia to rare pediatric spindle cell disorders such as infantile fibrosarcoma. Examples of solid tumors associated with specific translocations include the NUT-associated midline carcinoma, as well as several variants of salivary gland neoplasms. Translocation-associated neoplasms are found particularly in younger individuals. This test should be considered in all younger patients with difficult to diagnose neoplasms. Two recent cases highlight the practical utility of this clinical test in the diagnosis of difficult cases. In the first case, a 19-year old teenage boy had a soft tissue excision clinically thought to be a harmless branchial cleft cyst. However, histologic sections revealed a highly anaplastic neoplasm. Almost all immunohistochemical stains were negative. Some observers thought CD45 (LCA) was positive, while others thought the pattern of staining was non-specific. Similarly, some pathologists thought that ALK was positive in a peculiar weak cytoplasmic granular pattern, while White Paper others thought the staining represented nonspecific background staining. Based on the pattern of the ALK staining suggestive of an ALK-translocation involving the clathrin gene, B-cell clonalilty studies were performed to rule out an ALK-positive anaplastic large cell lymphoma. When these studies were negative, T-cell clonality studies were performed to rule out the possibility of an anaplastic large cell lymphoma. When these studies were negative, the case was studied by the Universal Fusion/Expression Profile. These studies revealed a rare fusion involving ALK and the SEC31-A gene, a fusion only previously reported in ALK-associated diffuse large B-cell lymphoma and ALKtranslocated pulmonary adenocarcinoma. These results led to a definitive diagnosis of ALK-translocated diffuse large B-cell lymphoma, validating the previous immunohistochemical studies. In the second case, a 72-year old woman presented with signs, symptoms, and laboratory findings typical for chronic myeloid leukemia. Yet, a real-time quantitative RT-PCR test designed to detect both the p210 and p190 BCR-ABL1 transcripts was negative. The peripheral blood sample was studied by the Universal Fusion/Expression Profile. This study revealed the presence of BCR-ABL1 fusion transcripts, however, the breakpoint on the ABL1 gene was found to involve exon 3 rather than the overwhelmingly common breakpoint involving exon 2. This rare variant had been reported previously in a case report. While it is not expected that the clinical response to therapy will differ from the more common fusion variant, it did indicate that the normal method to monitor patients with chronic myeloid leukemia, using the quantitative RT-PCR to detect p210 and p190 BCR-ABL1, would not be of use in this patient, as it does not cover exon 3, and only detects the common exon 2 translocations. With the development of new targeted therapies and a growing database of genomic data, the Universal Fusion/ Expression Profile has strong clinical applications beyond just diagnosis and should be considered for clinical research studies. Gene expression is currently being evaluated as a platform for identifying potential immunotherapy targets and the Universal Fusion/Expression Profile may have utility in this space. References 1. Vogelstein B, Papedopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. 2. Piskol R, Ramaswami G, Li JB. Reliable identification of genomic variants from RNA-seq data. Am J Hum Genet. 2013;93(4):641-651. 3. Stelow EB. A Review of NUT Midline Carcinoma. Head and Neck Pathology. 2011;5(1):31-35. doi:10.1007/s12105-010-0235-x. 4. Zeng Y, Feldman AL. Genetics of Anaplastic Large Cell Lymphoma. Leukemia & lymphoma. 2016;57(1):21-27. doi:10.3109/10428194.2015. 1064530. 5. Van Roosbroeck K, Cools J, Dierickx D, et al. ALK-positive large B-cell lymphomas with cryptic SEC31A-ALK and NPM1-ALK fusions. Haematologica. 2010;95(3):509-13. doi:10.3324/haematol.2009.014761. 6. Achkar W, Wafa A, Ali B, et al. A rare chronic myeloid leukemia case with Philadelphia chromosome, BCR-ABL e13a3 transcript and complex translocation involving four different chromosomes. Oncology Letters. 2010;1(5):797-800. doi:10.3892/ol_00000139. 7. Masucci G, Cesano A, Hawtin R, et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume 1 – pre-analytical and analytical validation. Journal for Immunotherapy of Cancer. 2016;4(76). doi:10.1186/s40425-016-0178-1. 12701 Commonwealth Dr., Suite 9 Fort Myers, FL 33913 Phone: 866.776.5907/ Fax: 239.690.4327 neogenomics.com © 2017 NeoGenomics Laboratories, Inc. All Rights Reserved. All other trademarks are the property of their respective owners. Rev. 032117