* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electron Transport and oxidative phosphorylation (ATP Synthesis)
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
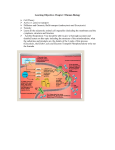
Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Abir Alghanouchi Biochemistry department Sciences college All of the reactions involved in cellular respiration can be grouped into three main stages ` Glycolysis – occurs in cytoplasm ` The Krebs cycle – occurs in matrix of mitochondria ` Electron transport – occurs across the mitochondrial membrane 2 ` Process in which ATP is formed as a result of transfer of electrons from NADH or FADH2 by a series of electron carriers ` The electron transport chain generates no ATP directly. Rather, its function is to break the large free energy drop from food to oxygen into a series of smaller steps that release energy During respiration, most energy flows from glucose ‐> NADH ‐> electron transport chain ‐> proton‐motive force ‐> ATP. 3 Phosphorylation process Oxidative process O2 einner membrane H 2O H+ ATP Synthase ADP+ Pi H+ outer membrane ATP intermembrane space matrix Figure: Essential features of oxidative phosphorylation ¾ Redox reactions of respiratory chain use electrons to reduce oxygen to water ¾ Energy generated moves protons from matrix to intermembrane space ¾ Inward movement of protons recovers this energy to promote formation of ATP in the matrix. 4 Protein complex Electron carrier Inner mitochondrial membrane Electron flow Electron transport chain ATP synthase 5 ATP yield ` Only 4 of 38 ATP ultimately produced by respiration of glucose are derived from substrate‐level phosphorylation (2 from glycolysis and 2 from TCA) ` The vast majority of the ATP (90%) comes from the energy in the electrons carried by NADH and FADH2 6 High-energy electrons carried mainly byNADH High-energy electronscarried by NADH Cytosol Mitochondrion Glycolysis Glucose 2 Pyruvic acid 2 Acetyl‐ CoA Krebs Cycle Electron Transport Maximum per glucose: by direct synthesis by direct synthesis by ATP synthase A Road Map for Cellular Respiration 7 ` Chemical reactions that transfer electrons from one substance to another are called oxidation‐reduction reactions ` REDOX short for oxidation‐reduction reactions 8 REDOX FACTS ` A:H A Reductant ' Oxidant + e‐ ` B B:H Oxidant + e‐ ' Reductant (acceptor) (donor) ` ` Both oxidation and reduction must occur simultaneously The reductant of one pair donates electrons and the oxidant of the other pair accepts the electrons Red1 (AH) + Ox2 (B) Î Ox1(A) + Red2(BH) 9 ` Electrons can move through a chain of donors and acceptors ` In the electron transport chain, electrons flow down a gradient ` Electrons move from a carrier with low reduction potential (high tendency to donate electrons) toward carriers with higher reduction potential (high tendency to accept electrons) 10 ` Potential (EO): measure of the tendency of oxidant to gain electrons, to become reduced, a potential energy. ` ∆EO: Standard reduction potential difference between two half reactions 11 Succinate Eo' = 0.03V ∆Eo' = 0.07V I NADH Eo' = -0.32V II Coenzyme Q Eo' = 0.10V ∆Eo' = 0.42V III ∆Eo' = 0.19V Cytochrome C Eo' = 0.29V IV ½ O2 Eo' = 0.82V ∆Eo' = 0.53V electron flow ¾ The components of the RC are arranged in order of increasing redox potential ¾ The ∆Eo′ values are the potential differences across the four complexes ( that span the mitochondrial inner membrane) 12 Succinate Eo' = 0.03V ∆Eo' = 0.07V I NADH Eo' = -0.32V II Coenzyme Q Eo' = 0.10V ∆Eo' = 0.42V III ∆Eo' = 0.19V Cytochrome C Eo' = 0.29V IV ½ O2 Eo' = 0.82V ∆Eo' = 0.53V electron flow ¾ The overall voltage drop from NADH E0′ = ‐(‐0.32 V) to O E0′ = +0.82 V is ∆Eº′ = 1.14 V 13 RC exists as four large, multi‐ subunit protein complexes ` ` ` ` The respiratory electron transport chain complex I is a NADH‐ ubiquinone reductase complex II is succinate dehydrogenase complex III is the ubiquinone ‐ cytochrome c reductase complex IV is cytochrome oxidase 14 Figure: Complex I of the respiratory chain that links NADH and coenzyme Q. ` NADH Dehydrogenase (NADH‐ubiquinone reductase) accepts 2e‐ from NADH and transfers them to ubiquinone (coenzyme Q), an electron carrier ` Uses two bound cofactors to accomplish this: FMN (Flavin mononucleotide) and 6 iron‐sulfur (Fe‐S) protein 15 Complex II: Succinate-CoQ reductase Prosthetic groups: FAD; Fe-S Succinate FAD SDH Fumarate ` ` ` FADH2 CoQ SDH is succinate dehydrogenase an enzyme of the citric acid cycle (associated with membrane) 2 e‐ transferred from succinate to CoQ 1 mole FADH2 produced 16 Electrons from complex I or II CoQ cyt b/cyt c1 Complex III: cytochrome reductase Prosthetic groups: heme b; heme c1; Fe-S cyt c Figure: Complex III of the respiratory chain linking CoQ and cytochrome C. ` Is composed of cytochome b, cytochrome C1 and iron sulphur proteins ` Accepts e‐ from coenzyme Q and transfers e‐ to cytochrome c coupled with the transfer of protons from the matrix to the intermembrane space 17 Figure: Complex IV ‐cytochrome oxidase‐ reducing oxygen to water ` Contains cytochromes a/a3 and 2 Cu ions involved in e‐ transfers ` Cytochrome oxidase passes electrons from cytochrome c through a series of heme groups and Cu ions to O2, reducing it to H2O (end product) 18 Coenzymes and cytochromes in the complexes act as e‐ donors & acceptors 19 ` Flavin MonoNucleotide (FMN), in Complex I, functions like FAD (which is an electron acceptor that helps electron transfer during Krebs Cycle and Electron Transport Chain in cellular respiration). ` iron‐sulfur (Fe‐S proteins): Fe‐S centers transfer e‐ in Complexes I, II and III ` Coenzyme Q (ubiquinone), lipid soluble, floats in the membrane and doesn’t require protein ` Cytochromes (b, c1, c, a, a3; contain heme): transfer e‐ in Complexes III and IV, Cytc is the only soluble cytochrome ` NAD+, FMN, CQ are carriers of e‐ and hydrogen while cytochromes are carriers of electrons only. 20 ` ATP‐synthase (complex V), present in the inner mitochondrial membrane, actually makes ATP from ADP and Pi. ` ATP used the energy of an existing proton gradient to power ATP synthesis. ◦ This proton gradient develops between the intermembrane space and the matrix. ◦ This concentration of H+ is the proton‐motive force. 21 ¾ The ATP synthase molecules are the only place that will allow H+ to diffuse back to the matrix ¾ This flow of H+ is used by the enzyme to generate ATP a process called chemiosmosis. ¾ Chemiosmosis: (osmos = push) is the oxidative phosphorylation that results in ATP production in the inner membrane of mitochondria. 22 Properties of ATP Synthase ` Multisubunit transmembrane protein ` Molecular mass = ~450 kDa ` Functional units ◦ F0: water‐insoluble transmembrane protein (up to 8 different subunits) ◦ F1: water‐soluble peripheral membrane protein (5 subunits) ,contains the catalytic site for ATP synthesis Flow of 3 protons through ATP synthase leads to phosphorylation of 1 ADP 23 ` Cytosolic NADH (glycolysis) must enter the mitochondria to fuel oxidative phosphorylation but NADH and NAD+ cannot diffuse across the inner mitochondrial membrane ` Two shuttle systems for reducing equivalents: 1. Glycerol phosphate shuttle: insect flight muscles 2. Malate Malate‐aspartate shuttle: predominant in liver and other mammalian tissues 24 25 ` In muscle and brain ` Each NADH converted to FADH2 inside mitochondrion ◦ FADH2 enters later in the electron transport chain ◦ Produces 2 ATP 26 27 ` In liver and heart ` NADH oxidized while reducing oxaloacetate to malate ◦ Malate dehydrogenase ` Malate crosses membrane 28 ` Malate reoxidized to oxaloacetate ◦ Malate dehydrogenase ◦ NAD+ reduced to NADH ` NADH via electron transport yields 3 ATP 29 Respiratory inhibitors ¾ These compounds prevent the passage of e‐ by binding a component of the ETC blocking the oxidation/reduction reaction 30 31 Complex designation Functional groups Function I– ----------NADH-Q reductase FMN (flavin ----------mono‐ nucleotide); Fe‐S oxidizes NADH to NAD+; Rotenone ----------------------transfers electrons to coenzyme Q II – SuccinateQ reductase ----------- FAD; Fe‐S oxidizes succinate to fumarate with reduction of FAD to --------------FADH2; electron transfer to CoQ III Cytochrome ----------reductase heme b; heme c 1; ---------Fe‐S transfers electrons between coenzyme Q -------------and cytochrome C (C becomes reduced) Antimycin A ---------- IV ----------C Cytochrome oxidase heme a‐a3; ---------Cu oxidizes cytochrome C; ------------reduces ½O 2 to H2O Carbon monoxide ---------Cyanide ---------- Inhibitors 32 ` Lippincot's Illustrated Reviews Biochemistry ` Lechinger's Principles of Biochemistry 4th edition. D. L. Nelson and M.M. Cox, Worth Publishers. ` Harpers illustrated biochemistry 25th edition. Robert K. Murray; Darly K. Granner: Victor W Rodwell ` www.rpi.edu/dept/bcbp/molbiochem ` www.med.ufl.edu/biochem/rcohen/rcohen.html ` http://courses.cm.utexas.edu/jrobertus/ch339k 33 34