* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Development of Mouse Hybridomas by Fusion of Myeloma Cells
Survey
Document related concepts
Duffy antigen system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Immune system wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
DNA vaccination wikipedia , lookup
Immunocontraception wikipedia , lookup
Innate immune system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adaptive immune system wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Transcript
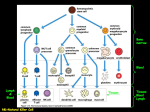
HYBRIDOMA AND HYBRIDOMICS Volume 22, Number 5, 2003 © Mary Ann Liebert, Inc. Short Communication Development of Mouse Hybridomas by Fusion of Myeloma Cells with Lymphocytes Derived from Spleen, Lymph Node, and Bone Marrow AYNUR BAŞALP and FATIMA YÜCEL ABSTRACT Since its discovery by Kohler and Milstein in 1975, hybridoma technology has found a wide use in almost every field of biology and medicine. A general and simple approach for developing monoclonal antibodies is to use splenocytes from immunized mice. In the present study, 10 fusion experiments were carried out to analyze the hybridization efficiencies of mouse myeloma cells with lymphocytes derived from spleen, lymph node, and bone marrow and we found a higher yield of antigen specific antibody producing hybridoma lines when the lymph nodes were used. INTRODUCTION K MILSTEIN ’S revolutionary work—hybridoma technology—made it possible to fuse mouse myeloma cells with B lymphocytes from immunized mice, thus resulting in antibody-producing somatic cell hybrids.(1) Since then, hybridoma technology has become widely adopted, and monoclonal antibodies (MAbs) have provided the solution to many biological problems. The most interesting and unique characteristics of a MAb is its specificity, not just for the immunizing protein but for a particular epitope region on it. In the past two decades, MAbs specific for numerous antigens (e.g., bacteria, viruses, parasites, tumor antigens, receptors, hormones, enzymes) have been generated, and MAbs have found widespread use in many areas of medical research, diagnostics, and therapeutics.(2–4) Although MAbs are powerful scientific tools and diagnostic reagents, there are still some unsolved practical problems. These technical problems include obtaining mAbs with high affinity and adequate specificity to very weak immunogens and antigens that are available in only very small amounts. In the traditional hybridoma technology, mice are immunized with desired antigen and once the animals are making OHLER AND a good antibody response, MAbs are generated by fusing the mouse splenocytes with a B cell myeloma from the same strain. In the investigation reported below, we used lymphocytes derived from spleen, lymph nodes, and bone marrow to enhance the chance of obtaining MAbs to weak or rare antigens. MATERIALS AND METHODS Antigens Hepatitis B surface antigens (HBsAg; Chemicon AG-850, AG-852, Temecula, CA), Verticillium dahliae, vascular endothelial growth factor–bovine serum albumin mixture (VEGFBSA; National Cancer Institute, Biological Resources Branch, Bethesda, MD), and anti-HBV IgG antibody were used as the immunogen. All antigens were emulsified with equal volume of Freund’s complete and incomplete mineral oil adjuvant, respectively, and immunizations were performed intraperitonally. Booster immunizations were done in phosphate buffered saline (PBS) by systemic routes. Research Institute for Genetic Engineering and Biotechnology (RIGEB), TÜBITAK, Gebze, Kocaeli, Turkey. 329 330 BAŞALP AND YÜCEL TABLE 1. COMPARISON OF THE NUMBER OF TOTAL HYBRID CELLS AND SPECIFIC ANTIBODY -PRODUCING HYBRID CELLS OBTAINED FROM SPLEEN AND LYMPH NODES Spleen Lymph node Number of total hybrid Number of specific hybrid Number of total hybrid Number of specific hybrid HBsAg Fusion 1 Fusion 2 210 426 1 — 83 51 2 — V. dahliae Fusion 1 Fusion 2 715 112 1 — 370 15 1 — VEGF-BSA a Fusion 1 Fusion 2 Fusion 3 262 146 179 — — — 31 125 39 1 4 1 Anti-HBV antibody Fusion 1 Fusion 2 Fusion 3 576 84 57 1 1 1 31 2 436 1 — 3 Antigen aAntibodies specific for VEGF and BSA. Fusion Fusion was carried out by some modifications of classical fusion protocols.(5) Briefly, lymphocytes from spleen, lymph nodes (bronchial, axillar, inguinal, popliteal, and intraperitonal), and bone marrow were fused with F0 (ATCC CRL 1646) mouse myeloma cells, and PEG 4000 was used as the fusing agent. Hybrid cells were selected by incubating cells in HAT medium, and positive clones were selected with ELISA. (6) Cloning and subcloning were performed as described.(7) ELISA Antigen-specific immune activity was determined by ELISA. Microtiter ELISA wells were coated with HBsAg (400 ng/mL), VEGF-BSA (400 ng/mL), Goat anti-HBV polyclonal antibody (1 mg/mL), and Verticillium dahliae (surface antigens, 1 mg/mL) in PBS at 4°C by overnight incubation, and unbound antigens were removed by washing with PBS 1 0.05% Tween-20. Free sites were blocked with 200 mL of 1% BSA or powdered milk for 1 h at 37°C and wells thoroughly washed. The diluted mouse serum or hybrid cell supernatant was added to each well and incubated for 1 h at 37°C. After washing, alkaline phosphatase conjugated goat antimouse IgG/IgM and polyvalent antibody (Sigma, St. Louis, MO, diluted as 1/4000, 1/1000 and 1/1000, respectively) were added. After 1 h of incubation at 37°C, the plates were washed, and PNPP (para nitrophenyl phosphate) at 1 mg/mL in substrate buffer (0.1 M glycine, pH 10.4, 1 mM ZnCl2, and 1 mM MgCl2) was added. The absorbance at 405 nm was read on a BioTec (Winooski, VT) EIA reader. RESULTS AND DISCUSSION Production of MAbs involves labor- and time-intensive procedures, lengthening their production time and ultimately their cost. Most laboratories engaged in the production of MAbs currently use lymphocytes from spleen. In the present study, a total of 10 fusions were carried out using lymphocytes from spleen and lymph nodes separately after immunizing animals with different antigens. Table 1 shows the number of total hybrid cells and antigen specific antibody producing hybrid cells from spleen and lymph nodes, respectively. When we compared the yield, the number of total hybrid cells seem higher in fusions performed using splenocytes. On the contrary, the number of hybridomas producing antibody of interest using lymph nodes was about two times higher than that of hybridomas with the conventional method using mouse spleen cells—four versus 10. The efficient use of lymph nodes for generating monoclonal antibodies with high specificity and affinity to antigens was also shown by other investigators.(8) Bynum and co-workers reported making high-affinity monoclonal antibodies by somatic TABLE 2. COMPARISON OF THE NUMBER OF TOTAL HYBRID CELLS AND SPECIFIC ANTIBODY -PRODUCING HYBRID CELLS BY USING BONE MARROW Bone marrow Number of total hybrid Number of specific hybrid V. dahliae Fusion 1 280 — Anti-HBV antibody Fusion 1 Fusion 2 Fusion 3 134 280 238 1a — — Antigen aAntibody has cross-reactivity. MOUSE HYBRIDOMAS WITH LYMPHOCYTES FROM SPLEEN, LYMPH NODE, AND BONE MARROW fusion of lymphocytes isolated from peripheral lymph nodes.(9) Sado et al. also used iliac lymph nodes for cell fusion and have shown that the production frequency of target hybridomas was about 10 times higher than that of a conventional spleen method.(10) Kimura et al. showed that fusion of popliteal lymph node lymphocytes produced hybridomas secreting antibodies to the peptide moiety of erythrocyte membrane protein. On the other hand, splenic lymphocyte fusion produced hybridomas secreting antibodies to the carbohydrate moiety of the same antigen.(11) Our data shown here is consistent with the previously reported results and reveal the generation of MAbs to antigens with higher specificity using lymph nodes. It is well established that, in mammals, lymphocytes circulate between bone marrow, spleen and lymph nodes and these organs are the major sites of antibody production in secondary immune response and thus, can be used as a source of activated B lymphocytes. Lymph nodes are small noduler aggregates of lymphoid tissue situated along lymphatic channels throughout the body and form part of a network that filters antigens from the interstitial tissue fluid.(12) Different classes of lymphocytes (T and B cells) and nonlymphoid accessory cells (dendritic cells, macrophages) are sequestered in particular areas of the node. Follicles are the B cell–rich areas of lymph nodes and T cells are located in parafollicular areas. Most of these T cells are CD4 T helper cells interacting with CD8 T cytotoxic cells. The paracortex contains many interdigitating antigen presenting cells (APC) expressing high levels of MHC class II receptors. When a foreign antigen is transported to the lymph node, T cells encounter the antigen and also dendritic cells, as well as other APCs, present the antigen to the T cell. Thus, the lymph nodes are the sites where T cell responses to lymph borne protein antigens are initiated. Bone marrow is a rich source of a variety of soluble factors (IL-1, IL-2, IL-6, IL-7, GMCSF, and IFN) and represents a special environment that supports and facilitates antibody production. Dilosa et al. have shown that germinal center B cells migrate to the bone marrow and produce large quantities of specific antibody associated with secondary immune response.(13) In four seperate fusions, bone marrow was isolated from immune animals, and lymphocytes were fused with mouse myeloma cells. Although primary hybridomas could be established from fusions with bone marrow–derived cells, only one out of 932 hybrid cells was production registered (Table 2). The results of this study indicate that the use of spleen, lymph nodes, and bone marrow in the same fusion after immunizing mice, especially with the rare or less immunodominant antigens, maximizes the chance of generating MAbs with the fewest attempts. This approach is a time- and labor-saving technique for making monoclonal antibodies. 331 ACKNOWLEDGMENTS This work was partly supported by TÜBITAK grant SBAG MAM1 102 S005 and SBAG 2496. REFERENCES 1. Kohler G, and Milstein C: Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256: 495–497. 2. Reichert JM: Monoclonal antibodies in the clinic. Nat Biotech 2001;19:819–822. 3. Glennie MJ, and Johnson PM: Clinical trials of antibody therapy. Immunol Today 2000;21:403–410. 4. Vora S: Monoclonal antibodies in enzyme research: present and potential applications. Anal Biochem 1985;144:307–318. 5. Galfre G, and Milstein C: Preperation of monoclonal antibodies: Strategies and procedures. Methods Enzymol 1983;73:3–46. 6. Doulliard JY, and Hoffman T: Enzyme-linked immunosorbent assay for screening monoclonal antibody production using enzymelabeled second antibody. Methods Enzymol 1983;92:168–174. 7. Reid LCM: Cloning. Methods Enzymol 1979;58:152–164. 8. Jahn S, Grunow R, Kiessig ST, Specht U, Matthes H, Hiepe F, and Hlin Von Baehr R: Establishment of human Ig producing heterohybridomas by fusion of mouse myeloma cells with human lymphocytes derived from peripheral blood, bone marrow, spleen, lymph node and synovial fluid. Effect of polyclonal prestimulation and cryopreservation. J Immunol Methods 1988;107:59–66. 9. Bynum J, Andrews JL, Ellis B, Kull FC, Austin EA, and Kilpatrick K: Development of class-switched, affinity-maturated monoclonala ntibodies following a 7-day immunization schedule. Hybridoma 1999;8:407–411. 10. Sado Y, and Okigaki T: A novel method for production of monoclonal antibodies. Evaluation and expectation of the rat lymph node method in cell and molecular biology. Cell Biol Int 1996;20:7–14. 11. Kimura A, Nakashima R, Inoeu H, Yasuda S, Ikemoto E, and Nishimoto TT: Differential production of monoclonal antibodies to carbohydrate moity or peptides moiety of glycoproteins by different routes of immunization. Hybrdioma 1998;17:245–250. 12. Abbas AK, Lichtman AH, and Pober JS: Cellular and Molecular Immunology. Saunders, Philadelphia, 2000, pp. 17–38. 13. Dilosa RM, Kunihiko M, Masuda A, Szakal AS, and Tew JG: Germinal center B cells and antibody production in the bone marrow. J Immunol 1991;146:4071–4077. Adress reprint requests to: Aynur Başalp, Ph.D. TÜBÌTAK—RIGEB 41470 Gebze, Kocaeli, Turkey E-mail: [email protected] Received for publication June 16, 2003. Accepted for publication July 21, 2003.