* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Genetic Differences in Endothelial Cells May Determine
Genome evolution wikipedia , lookup
Minimal genome wikipedia , lookup
Gene therapy wikipedia , lookup
Ridge (biology) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Behavioural genetics wikipedia , lookup
Pathogenomics wikipedia , lookup
Population genetics wikipedia , lookup
Heritability of IQ wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene expression programming wikipedia , lookup
Genomic imprinting wikipedia , lookup
Medical genetics wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Human genetic variation wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene expression profiling wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Designer baby wikipedia , lookup
Microevolution wikipedia , lookup
History of genetic engineering wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
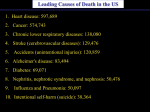
Genetic Differences in Endothelial Cells May Determine Atherosclerosis Susceptibility Jan L. Breslow, MD A Downloaded from http://circ.ahajournals.org/ by guest on June 18, 2017 from fatty streaks to fibrous plaques to complicated lesions.3 In the diet-induced mouse atherosclerosis model, lesion formation is largely limited to the aortic root, even after feeding mice the Paigen diet for 14 weeks to 9 months. The lesions are often small (only several hundred to a few thousand square microns), and they consist almost entirely of macrophage foam cells, with little evidence of progression to fibrous plaques involving smooth muscle cells. Another concern with the diet-induced model is that the unphysiological diet required to produce lesions (extremely high cholesterol content and the presence of cholic acid) causes a chronic inflammatory state, with the expression of inflammatory and oxidative stress genes (ie, MCP-1, CSFs, heme oxygenase, SAA, and NFB activation) in the atherosclerosis-sensitive mouse strains.4 Although the inflammation correlates with atherosclerosis in this model,5 it is possible that some of the genetic differences between susceptible and resistant mouse strains pertain to the diet used, rather than the atherogenic process as it is observed on Western diets. The diet-induced mouse atherosclerosis model has been used in an attempt to identify genes for atherosclerosis susceptibility. In Paigen et al’s6,7 original work, a locus named Ath-1 was identified. Ath-1 denotes a phenotype in which susceptible, but not resistant, mice respond to the high-fat, high-cholesterol, cholate-containing diet with increased fatty streak lesions at the aortic root and reduced HDL cholesterol levels. In these studies, they used recombinant inbred (RI) strains derived from C57Bl/6J (susceptible) and C3H/HeJ (resistant) mice, as well as a cross between C57Bl/6J and C3H/HeJ mice. This work indicated a locus regulating both the atherosclerosis and the HDL cholesterol phenotypes on mouse chromosome 1, near but separate from the apolipoprotein A-II gene. In subsequent work in crosses with a number of mouse strains, other atherosclerosis loci were identified; they were designated Ath-2 thru Ath-8.8 –12 Ath-3 has been mapped to chromosome 7 and Ath-6 to chromosome 12, but the others have not been mapped. Another group was not able to confirm the map position of Ath-1 in a reanalysis of the original RI strains that were used or in a quantitative trait locus mapping experiment.13,14 None of the genes corresponding to Ath-1 through Ath-8 have been identified. An antioxidant gene was identified in the region of Ath-1 (Aop2),15 but Paigen et al have not provided proof that this gene is indeed Ath-1. In a recent article describing Ath-8, the authors (including Paigen) discussed the difficulty of finding genes in the diet-induced model. They noted that “it is common to find no lesions in an SM (atherosclerosis sensitive) mouse, despite its having been fed an atherogenic diet. The fact that there is therosclerotic cardiovascular disease is a complex genetic disorder that involves many genes and has significant gene-environment interactions. The extensive study of candidate genes in pathways relevant to atherosclerotic cardiovascular disease risk factors has had limited success in explaining the population’s susceptibility to this disease. With the advent of the Genome Program, it is now becoming possible to use positional cloning techniques to reveal new genes involved in atherosclerosis susceptibility. Studies in human populations are underway, but they may be difficult because of the heterogeneity of human populations and the very real possibility that large numbers of genes are involved, each with very small effects. The probable difficulties of human genetic studies strongly suggest that parallel approaches should be undertaken to identify genes for atherosclerosis susceptibility by positional cloning in animal models. Genes identified through the study of animal models can be used to identify human homologues. These can be used to search for mutations associated with disease in human populations and/or to identify important pathways involved in atherogenesis that might lend themselves to the development of novel therapeutic approaches. See p 75 The most ideal mammal for such studies is the laboratory mouse.1 Mice are small (25 to 40 g) and have short generation times (9 to 10 weeks) and large litter sizes (5 to 10 pups); in addition, there are many inbred strains available. However, laboratory strains of mice fed the normal chow diet do not develop atherosclerotic lesions. To circumvent this problem, Paigen et al2 proposed a model in which mice are fed a diet containing 15% fat, 1.25% cholesterol, and 0.5% cholic acid to induce lesions. They used this diet to study 10 inbred mouse strains and found large differences in atherosclerosis susceptibility that did not correlate well with total plasma cholesterol levels, implying other factors were involved in susceptibility to this diet. Unfortunately, serious limitations of the diet-induced mouse atherosclerosis model exist. Human atherosclerotic lesions typically occur at vessel branch points and progress The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association. From the Laboratory of Biochemical Genetics and Metabolism, Rockefeller University, New York, NY. Correspondence to Jan L. Breslow, MD, Laboratory of Biochemical Genetics and Metabolism, The Rockefeller University, 1230 York Ave, Box 179, New York, NY 10021-6399. E-mail [email protected] (Circulation. 2000;102:5-6.) © 2000 American Heart Association, Inc. Circulation is available at http://www.circulationaha.org 5 6 Circulation July 4, 2000 Downloaded from http://circ.ahajournals.org/ by guest on June 18, 2017 incomplete penetrance reduces the power of linkage analysis by weakening the correlation between genotype and phenotype.”12 The small lesion size in the diet-induced mouse atherosclerosis model and the incomplete penetrance of lesions add to the difficulty of isolating genes for atherosclerosis susceptibility using this model. Thus, it is clear that new approaches will be needed with this model to identify the genes responsible for the differences observed in susceptibility to atherosclerosis. The article in the current issue of Circulation by Shi et al16 contributes to this goal. In this study, the authors build on previous work with the diet-induced mouse atherosclerosis model and take it in a new direction. They report a method for culturing endothelial cells from the mouse thoracic aorta by an explant technique. They then show that minimally modified LDL, a mildly oxidized form of LDL, induced higher levels of inflammatory gene expression in C57Bl/6J-derived compared with C3H/HeJ-derived cultured aortic endothelial cells. These are the same 2 mouse strains originally used by Paigen et al, which are atherosclerosis sensitive and resistant, respectively. The authors conclude that their experiments “provide strong evidence that genetic factors in atherosclerosis act at the level of the vessel wall.” This article by Shi et al16 is important from several points of view. It provides the first direct proof that factors operating in the vessel wall, particularly endothelial cells, can serve as atherosclerosis modifiers. This is reminiscent of clinical experience in which patients with the same risk factors have vastly different extents of disease, and it suggests a possible mechanism for these clinical observations. The article demonstrates that the induction of an inflammation-related phenotype relevant to atherosclerosis susceptibility is preserved ex vivo in tissue culture cells and is independent of the relatively toxic diet used in the diet-induced mouse atherosclerosis model. The article also provides an in vitro tissue culture system to compare endothelial cells from C57Bl/6J and C3H/HeJ mice for differences in minimally modified LDL binding and signal transduction. These cultures allow one to design experiments to identify the pathway, specific molecules, and perhaps even genes underlying the difference in atherosclerosis susceptibility between the 2 strains. Finally, the article by Shi et al16 provides a secondary marker for atherosclerosis susceptibility that can be scored in quantitative trait locus mapping of atherosclerosis susceptibility genes. Atherosclerosis is a complex phenotype, and it may be more revealing in future mouse crosses using the diet-induced mouse model to score secondary markers that might have a simpler inheritance pattern. It may also be useful to score endothelial responsiveness to minimally modified LDL in ongoing crosses with other mouse atherosclerosis models, such as the apoE knockout mouse.17 Shi et al16 provide another important step in the process of using the powerful tools of mouse genetics to help unravel the mysteries of atherosclerosis, a very complex human genetic disease. References 1. Silver LM. Mouse Genetics. New York: Oxford University Press; 1995:362. 2. Paigen B, Morrow A, Brandon C, et al. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57: 65–73. 3. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med, 1999; 340:115–126. 4. Liao F, Andalibi A, deBeer FC, et al. Genetic control of inflammatory gene induction and NF-kappa B-like transcription factor activation in response to an atherogenic diet in mice. J Clin Invest. 1993;91: 2572–2579. 5. Liao F, Andalibi A, Qiao JH, et al. Genetic evidence for a common pathway mediating oxidative stress, inflammatory gene induction, and aortic fatty streak formation in mice. J Clin Invest. 1994;94:877– 884. 6. Paigen B, Mitchell D, Reue K, et al. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci U S A. 1987;84:3763–3767. 7. Paigen B, Albee D, Holmes PA, et al. Genetic analysis of murine strains C57BL/6J and C3H/HeJ to confirm the map position of Ath-1, a gene determining atherosclerosis susceptibility. Biochem Genet. 1987;25: 501–511. 8. Paigen B, Nesbitt MN, Mitchell D, et al. Ath-2, a second gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Genetics. 1989;122:163–168. 9. Stewart-Phillips JL, Lough J, Skamene E. ATH-3, a new gene for atherosclerosis in the mouse. Clin Invest Med. 1989;12:121–126. 10. Paigen B. Genetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am J Clin Nutr. 1995;62:458S– 462S. 11. Mu JL, Naggert JK, Svenson KL, et al. Quantitative trait loci analysis for the differences in susceptibility to atherosclerosis and diabetes between inbred mouse strains C57BL/6J and C57BLKS/J. J Lipid Res. 1999;40: 1328 –1335. 12. Pitman WA, Hunt MH, McFarland C, et al. Genetic analysis of the difference in diet-induced atherosclerosis between the inbred mouse strains SM/J and NZB/BINJ. Arterioscler Thromb Vasc Biol. 1998;18: 615– 620. 13. Hyman RW, Frank S, Warden CH, et al. Quantitative trait locus analysis of susceptibility to diet-induced atherosclerosis in recombinant inbred mice. Biochem Genet. 1994;32:397– 407. 14. Machleder D, Ivandic B, Welch C, et al. Complex genetic control of HDL levels in mice in response to an atherogenic diet: coordinate regulation of HDL levels and bile acid metabolism. J Clin Invest. 1997;99:1406 –1419. 15. Phelan SA, Johnson KA, Beier DR, et al. Characterization of the murine gene encoding Aop2 (antioxidant protein 2) and identification of two highly related genes. Genomics. 1998;54:132–139. 16. Shi W, Haberland ME, Jien M-L, et al. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;101:75– 81. 17. Dansky H, Charlton SA, Sikes JL, et al. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1960 –1968. KEY WORDS: 䡲 atherosclerosis Editorials 䡲 endothelium 䡲 inflammation 䡲 genetics Genetic Differences in Endothelial Cells May Determine Atherosclerosis Susceptibility Jan L. Breslow Circulation. 2000;102:5-6 doi: 10.1161/01.CIR.102.1.5 Downloaded from http://circ.ahajournals.org/ by guest on June 18, 2017 Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2000 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/102/1/5 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/