* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Generation of Monoclonal Antibodies to Cryptic Collagen Sites by
Survey
Document related concepts
Gluten immunochemistry wikipedia , lookup
Immune system wikipedia , lookup
DNA vaccination wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Immunocontraception wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Molecular mimicry wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Transcript
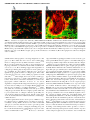
HYBRIDOMA Volume 19, Number 5, 2000 Mary Ann Liebert, Inc. Generation of Monoclonal Antibodies to Cryptic Collagen Sites by Using Subtractive Immunization JINGSONG XU, DOROTHY RODRIGUEZ, JENNY J. KIM, and PETER C. BROOKS ABSTRACT The extracellular matrix (ECM) plays a fundamental role in the regulation of normal and pathological processes. The most abundantly expressed component found in the ECM is collagen. Triple helical collagen is known to be highly resistant to proteolytic cleavage except by members of the matrix metalloproteinase (MMP) family of enzymes. To date little is known concerning the biochemical consequences of collagen metabolism on human diseases. This is due in part to the lack of specific reagents that can distinguish between proteolyzed and triple helical forms of collagen. Here we used the technique of Subtractive Immunization (SI) to generate two unique monoclonal antibodies (MAbs HUIV26 and HUI77) that react with denatured and proteolyzed forms of collagen, but show little if any reaction with triple helical collagen. Importantly, HUIV26 and HUI77 react with cryptic sites within the ECM of human melanoma tumors, demonstrating their utility for immunohistochemical analysis in vivo. Thus, the generation of these novel MAbs not only identify specific cryptic epitopes within triple helical collagen, but also provide important new reagents for studying the roles of collagen remodeling in normal as well as pathological processes. INTRODUCTION H mATRIX (ECM) was thought to function mainly to provide mechanical support for cells and tissues. However, it is now well accepted that the ECM contains a wealth of biochemical information, which helps to regulate nearly all aspects of cellular behavior.(1–5) One of the most abundantly expressed proteins that help comprise the ECM is collagen.(6,7) Studies have identified at least 30 separate genes that code for distinct chains of collagen, and to date at least 19 different types of collagen have been identified.(6–8) Collagen is composed of three chains organized in a triple helical fashion. This triple helical structure, in conjunction with its capacity to bind numerous ECM proteins contributes to its mechanical strength and resistance to proteolytic attack.(6–8) Thus, collagen is uniquely suited for its role as a major structural component of the ECM. Triple helical collagen can be cleaved by a family of proteolytic enzymes termed Matrix Metalloproteinases (MMPs).(9,10) Specific members of this family have the ability to cleave triple helical collagen into characteristic one-quarter and three-quarter sized fragments.(9,10) These proteolyzed forms of collagen ISTORICALLY THE EXTRACELLULAR are then rendered susceptible to further degradation by broadspectrum proteases.(9,10) While cellular interactions with intact triple helical collagen is known to regulate cellular behavior via interactions with specific integrin receptors, proteolytic remodeling of collagen is also thought to be of great importance in the regulation of a number of disease processes, including tumor growth and metastasis.(11–13) To this end, immunological reagents such as monoclonal and polyclonal antibodies have been developed to study the expression, and distribution of collagen in vitro and in vivo.(14–16) However, the majority of these antibodies recognize epitopes within the triple helical or noncollagenous domains of collagen. In fact, few reagents have been developed that can selectively bind to proteolyzed or denatured collagen yet have no reactivity to native triple helical collagen. A hallmark of invasive cellular behavior is the capacity of cells to proteolytically remodel their immediate microenvironment. Thus, monoclonal antibodies (MAbs) that specifically recognize distinct epitope that are selectively exposed following collagen proteolysis would be of great use in studying the roles of collagen metabolism in invasive cellular behavior. A major impediment to the development of MAbs directed University of Southern California Keck School of Medicine, Department of Biochemistry and Molecular Biology, Norris Cancer Center, 1441 Eastlake Ave, Los Angeles, CA 90033. 375 376 XU ET AL. to cryptic sites within collagen is associated with the immunodominant epitopes found within its triple helical and noncollagenous domains. These major structural epitopes likely dominate the immune response. In fact, the majority of MAbs developed to date are directed to epitopes normally exposed within the intact collagen molecule. Thus, establishment of a protocol to shift the immune response away from the immu-nodominant epitopes toward cryptic sites that are only exposed following proteolysis would be of great use. To this end, we have utilized a unique immunological tolerization approach called Subtractive Immunization (SI)(17,18) to develop a set of novel MAbs that specifically react with cryptic epitopes that are normally hidden within the three-dimensional structure of collagen. Here we describe the generation and characterization of MAbs termed HUIV26 and HUI77. These MAbs specifically recognize denatured and proteolyzed collagen, but exhibit little if any reactivity to triple helical collagen. Moreover, these unique MAbs are highly specific to distinct forms of collagen and do not crossreact with other ECM proteins. Interestingly, these MAbs were shown to be useful for a number of biochemical assays including, solid phase enzyme-linked immunoadsorbent assay (ELISA), Western blotting, and immunofluorescence analysis of frozen tissues. Thus, the binding characteristics of MAbs HUIV26 and HUI77 not only help to define a new set of cryptic epitopes within distinct forms of collagen, but provide immunological reagents for studying the roles of collagen degradation in normal as well as pathological processes. MATERIALS AND METHODS Reagents and chemicals Purified human collagen types I and IV were obtained from UBI (Lake Placid, NY), types II, III, and V were purchased from Chemicon (Temecula, CA). Purified Fibronectin, Laminin, and Fibrinogen were obtained from Sigma (St. Louis, MO). Vitronectin was a kind gift from Dr. David Cheresh, Scripps Research Institute (La Jolla, CA). OCT was obtained from VWR (San Francisco, CA). OPD substrate was obtained from Sigma (St. Louis, MO). Polyclonal antibody to Factor VIII-related antigen was obtained from BioGenex (San Ramon, CA). Rhodamine-conjugated goat anti-rabbit IgG, fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and peroxidase labeled goat anti-mouse IgG were purchased from BioSource International (Camarillo, CA). Cyclophosphamide was obtained from Sigma (St. Louis, MO). Cells and cell culture SP-20 myeloma cells were purchased from ATCC (Manassas, VA). SP-20 myeloma cells were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1%, Pyruvate and Glutamate, and Pen-Strep. Cells were maintained as subconfluent cultures until used. Subtractive immunization The SI protocol was performed as previously described with some modifications.(18) Female BALb/c mice, 6 to 8 weeks old, were obtained from B&K (Fremont, CA). On Day 1, mice were inoculated with 200 mg of triple helical human collagen type I or IV. On Days 2 and 3, mice were injected i.p. with 150 mg/kg cyclophosphamide in sterile phosphate-buffered saline (PBS). Following the cycle of cyclophosphamide injections, the mice were allowed to recover for 2 weeks. This tolerization procedure was repeated a total of three times. Following the three cycles of tolerization to triple helical collagen, mice were injected i.p. with thermally denatured (boiled 12 min) collagen at a concentration of 200 mg per mouse. This immunization procedure was repeated once every 3 weeks for a total of four injections. At the end of the injection cycle, sera were collected and screened for differential reactivity to triple helical and thermally denatured collagen. The mice with the greatest immunoreactivity to thermally denatured collagen, as compared with triple helical collagen, were selected for the fusion. Production of hybridomas Hybridoma fusions were completed by standard techniques(18) Briefly, the selected mice were sacrificed and the spleens and associated lymph nodes were removed washed and the spleen cells isolated. The spleen cells were fused (4:1 spleen cells to myeloma cells) with mouse myeloma cell line SP20. The cells were plated at a density of 2.5 3 104 cells in 100 mL per well of hipoxantine, aminopterine, and thymidine (HAT) medium. The Hybridoma cultures were allowed to grow for at least 7 days before analysis. Conditioned medium from hybridoma cultures were screen by solid-phase ELISA assays for reactivity to either triple helical and thermally denatured collagen types I and IV. Two clones were selected for further analysis. These hybridomas were subcloned by limiting dilution and isotyped by standard procedures.(18) Both MAbs HUIV26 and HUI77 were determined to be IgMs with a Kappa light chain. MAb purification Hybridoma clones HUIV26 and HUI77 were injected in pristine primed Balb/c mice to produce acites fluid. Acites fluid was cleared by centrifugation and applied to an Immunopure Mannose Binding Column for purification of IgMs, (Pierce, Rockford, IL). Antibody purifications were carried out according to the manufacturers instructions. Solid phase ELISA assays Purified ECM proteins (25 mg/mL in PBS) were coated (50 mL/well) in 96-well microtiter plates for 16 h at 4°C. The plates were washed with PBS and blocked with 1% protease free bovine serum albumin (BSA) in PBS (100 mL/well). Purified MAbs HUIV26 and HUI77 (1 mg/mL) in a total volume of 50 mL were incubated for 1 h at 37°C. Plates were washed as before and incubated with peroxidase-conjugated goat anti-mouse IgG 1 IgM at a dilution of 1:3000 in 1% BSA in PBS. Finally, plates were washed and developed by the addition of OPD (0.4 mg/mL) in 80-mM citrate-phosphate buffer (pH 5.0). Color reaction was stopped by the addition of 25 mL of 4N H2SO4. Immune reactivity was quantified by measuring the optical density (O.D.) at a wave length of 490 nm with a microtiter plate reader. All data were corrected for nonspecific binding to BSA. In addition, the O.D.s were also corrected for nonspecific binding of the secondary antibody. GENERATION OF MAbs TO CRYPTIC SITES IN COLLAGEN Proteolyzed collagen ELISA assay Microtiter wells were coated with 25 mg/mL of triple helical collagen type IV, as described above. HUVEC conditioned medium was added to the wells (100 mL) and allowed to incubate for 1, 6, and 24 h at 37°C. The wells were extensively washed with PBS/ethylenediaminetetraacetic acid (EDTA) and blocked with BSA to prevent nonspecific binding and ELISAs were completed as described above. In control experiments, wells were coated with HUVEC-conditioned medium in the absence of collagen and no reactivity with MAbs HUIV26 or HUI77 was detected. To confirm the exposure of cryptic epitopes was dependent on proteolytic activity, protease inhibitors including Aprotinin (10 mg/mL) and EDTA (50 mM) was added to the HUVEC-conditioned medium during the incubation reactions. Under these conditions, HUIV26 and HUI77 showed little if any reactivity. Finally, little if any changes in the amount of immobilized collagen on the microtiter wells were detected following proteolysis as detected by a polyclonal antibodies directed to triple helical and denatured collagen. Western blot analysis Immunoblotting was performed essentially as described with some modifications.(19) Briefly, purified human collagen types I or IV were separated by electrophoresis through a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE) gel. Separated proteins were transferred to nitrocellulose membranes and probed with either MAbs HUIV26 or HUI77 (1.0 mg/mL). Antibody reactivity was detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody and visualized with enhanced chemiluminescent substrate PS-3 according to the manufactures instructions. Immunofluorescence analysis Immunofluorescent analysis was performed essentially as described with minor modifications.(20) Tissue biopsies from metastatic human melanomas were embedded in OCT and snap frozen in liquid nitrogen. Frozen tissue sections were cut (4.0 mm) with the use of a cryostat, as described previously.(20) Tissue sections were next blocked with 2.5% BSA in PBS for 1 h at room temperature. The tissues were washed and incubated with MAb HUIV26 or HUI77 (100 mg/mL) and either polyclonal antibodies directed to av integrin subunit (1:1000 dilution) or Factor VIII related antigen (1:50 dilution) in 1.0% BSA in PBS for 2 h at 37°C. Tissue sections were washed 53 with PBS for 5 min, followed by incubation with FITC-conjugated goat anti-mouse and Rhodamine-conjugated goat anti-rabbit secondary antibodies (1:400 dilution in BSA in PBS) for 1 hr. Tissue sections were washed as before and mounted with antifade medium.(20) Immunofluorescence photomicrographs were taken at a magnification of 2003. RESULTS Use of subtractive immunization to suppress the immune response to dominant epitopes in triple helical collagen While numerous studies have documented structural cleavage of purified ECM proteins in vitro, little direct evidence is 377 available concerning proteolytic cleavage of ECM proteins within the in vivo microenvironment. This is due in large part to the lack of reagents that can distinguish between the native and proteolyzed forms of ECM proteins. To this end, we have utilized SI to develop MAbs that recognize proteolyzed and denatured forms of genetically distinct collagen molecules, but have little if any reaction with triple helical collagen. The SI protocol involves tolerizing mice to the major immunodominant epitopes present on triple helical collagen (Fig. 1). The tolerization steps were performed a total of three times to ensure suppression of the immune response to the major immunodominant epitopes accessible within triple helical collagen molecules. Following immune suppression with cyclophosphamide, mice were injected with either denatured collagen types I or IV, to stimulate an immune response to sites are uniquely exposed following denaturation (Fig. 1). Analysis of mouse sera for immunoreactivity to triple helical and denatured collagen To evaluate whether the subtractive immunization procedure was effective in shifting the immune response toward the generation of antibodies that would reacted with denatured forms of collagen, solid-phase ELISA assays were carried out. Microtiter plates were coated with either triple helical or thermally denatured human collagen types I or IV. The microtiter wells were blocked with BSA to prevent nonspecific interactions. Sera from experimentally manipulated mice were tested for reactivity with either triple helical and denatured collagen types I and IV. As shown in Table 1, sera from tolerized mice injected with denatured collagen type I showed an approximate 2- to 7-fold increase in reactivity with denatured collagen type I as compared with triple helical collagen type I. Moreover, sera from tolerized mice injected with denatured collagen type IV showed an approximate 2- to 12-fold increase in reactivity with denatured collagen type IV as compared with triple helical collagen type IV. These findings suggest that use of the SI procedure can result in a shift in the immune response in favor of the generation of antibodies directed to cryptic epitopes exposed following thermal denaturation. Based on these findings, mice numbers 2 and 5 were selected for use in the fusion for the generation of hybridomas. Characterization of MAbs HUIV26 and HUI77 binding specificity to ECM proteins Standard hybridoma techniques were carried out for two separate fusions for the generation on hybridomas producing antibodies directed to either denatured collagen types I or IV.(18,21) Hybridoma clones derived from each fusion were screened for reactivity with triple helical and denatured collagen types I and IV by solid-phase ELISA. Based on these studies, two MAbs termed HUIV26 and HUI77 were subcloned by limiting dilution and chosen for further analysis. Isotyping analysis of these MAbs showed that both were IgMs with a kappa light chain (data not shown). To evaluate the binding specificity of MAbs HUIV26 and HUI77 to distinct ECM proteins, solid-phase ELISA assays were performed. As shown in Fig. 2A, MAb HUIV26 recognized thermally denatured collagen type IV, while showing little if any reactivity to triple helical collagen type IV. Moreover, MAb HUIV26 recognized denatured colla- 378 XU ET AL. FIG. 1. Schematic representation of the Subtractive Immunization (SI) protocol. The SI protocol can be organized into four steps: Step I involves the tolerization of mice by completing three cycles of injections of the antigen to be tolerized to (triple helical collagen), followed by cyclophosphamide injections at 24 and 48 h following the antigen. Step II involves immunizing the mice with thermally denatured collagen. The third step includes fusion of the spleen cells from selected mice with myeloma cells. The final step includes screening individual hybridoma clones for those that produce antibodies that are selective for thermally denatured collagen as compared with triple helical collagen. gen type IV in a dose-dependent and saturable manner (data not shown). Importantly, MAb HUIV26 showed no cross-reactivity to other ECM proteins, including fibronectin, fibrinogen, laminin, or vitronectin. In similar experiments, MAb HUI77 specifically bound thermally denatured collagen type I, while showing little if any binding to triple helical collagen type I (Fig. 2B). In addition, HUI77 also reacted with denatured col- lagen type I in a dose-dependent manner (data not shown). MAb HUI77 also showed no reactivity to other ECM proteins, including fibronectin, fibrinogen, laminin, or vitronectin. Neither of these MAbs showed any reactivity with thermally denatured forms of other ECM proteins, further demonstrating their specificity for collagen (data not shown). Finally, MAbs HUIV26 and HUI77 were also shown to recognize denatured collagen TABLE 1. ELISA SCREEN OF MOUSE SERA Mouse number Thermally denatured collage I Triple helical collagen I ELISA ratio denatured/triple helical Mouse #1 Mouse #2 0.254 (6 0.002) 0.264 (6 0.009) 0.110 (6 0.002) 0.036 (6 0.006) 2.3 7.3 Mouse number Thermally denatured collage I Triple helical collagen I ELISA ratio denatured/triple helical Mouse #3 Mouse #4 Mouse #5 0.420 (6 0.011) 0.455 (6 0.032) 0.526 (6 0.061) 0.098 (6 0.008) 0.178 (6 0.063) 0.044 (6 0.099) 14.2 12.6 12.0 Microtiter wells were coated with either triple helical or denatured collagen type I or IV. Sera (1: 100 dilution) form experimentally treated mice were examined for reactivity to collagen in solid phase ELISA assays. Data represent mean optical density (O.D.) 6 standard deviations from triplicate wells. 379 GENERATION OF MAbs TO CRYPTIC SITES IN COLLAGEN A C B FIG. 2. ELISA analysis of MAb reactivity to purified ECM protein. Microtiter wells were coated with 25 mg/mL of purified ECM proteins collagen types I and IV, fibrinogen, fibronectin, laminin, and vitronectin. Purified MAb antibodies HUIV26 (A) and HUI77 (B) were added to the wells (1.0 mg/mL) in a total volume of 50 mL and allowed to incubate for 1 h. The wells were washed and incubated with peroxidase labeled goat anti-mouse secondary antibody. Immunoreactivity was detected following addition of OPD substrate by measuring the optical density (O.D.) at a length of 490 nm. All data were corrected for nonspecific reactivity with secondary antibody. Data bars represent the mean O.D. 6 standard deviation from triplicate wells. (C). Western blot analysis of purified collagen type I and IV using MAbs HUIV26 and HUI77. Top panel, purified collagen probed with MAb HUIV26. Bottom panel, purified collagen probed with MAb HUI77. Lane 1, purified collagen type 1. Lane 2, purified collagen type IV. type I and type IV in Western blot analysis of purified collagen (Fig. 2C). While MAb HUIV26 recognized its epitope within one a-chain of collagen type IV (Fig. 2C, top panel), MAb HUI77 recognized epitopes within a-chains of collagen types I and IV (Fig. 2C, bottom panel). These results confirm the specificity and utility of these MAbs in Western blotting (Fig. 2C). Taken together, these findings suggest the MAbs HUIV26 and HUI77 identify unique cryptic epitopes of colla- gen, which are specifically exposed following denaturation, but which are normally hidden within the triple helical molecule. Differential reactivity of MAbs HUIV26 and HUI77 to genetically distinct forms of collagen Collagen represents the most abundantly expressed ECM protein in the body.(6–8) In fact, at least 19 distinct forms of col- 380 lagen have been identified.(6–8) Therefore, to determine whether the cryptic epitopes defined by MAbs HUIV26 and HUI77 were specific to collagen types I and IV or whether these epitopes were present within other collagen molecules, solid-phase ELISA assays were performed. To facilitate these studies microtiter plates were coated with triple helical or thermally denatured collagen types I, II, III, IV, and V. The microtiter wells were blocked with BSA to prevent nonspecific binding and purified MAb HUIV26 and HUI77 were tested for reactivity. As shown in Fig. 3A, MAb HUIV26 readily bound to thermally denatured collagen type IV while showing little if any reactivity to triple helical collagen. Importantly, MAb HUIV26 showed little if any reactivity with other distinct forms of collagen, including types I, II, III, or V. In contrast, MAb HUI77 reacted with the denatured forms of all the genetically distinct forms of collagen tested (Fig. 3B). However, MAb HUI77 showed little if any reactivity to the triple helical forms of these collagen molecules. Taken together these findings indicate that while the HUIV26 epitope appears specific for collagen type IV, the HUI77 cryptic epitope is present within all the genetically distinct forms of collagen tested. Exposure of the HUIV26 and HUI77 cryptic epitopes following proteolysis Our previous studies have indicated that thermal denaturation can expose the cryptic epitopes defined by MAbs HUIV26 and HUI77. However, it is possible that structural changes conferred by thermal denaturation may be different than that resulting from proteolytic cleavage. Moreover, proteolytic cleavage of collagen likely represent a more physiologically relevant mechanism of ECM remodeling than thermal denaturation. Therefore, we examined whether the cryptic epitopes defined by MAbs HUIV26 and HUI77 could be exposed by proteolytic activity. To facilitate these studies, mictotiter wells were coated with triple helical collagen type IV. The coated wells were next incubated with concentrated serum free Human Umbilical Vein Endothelial Cell (HUVEC) conditioned medium for 1, 6, and 24 h. Endothelial cell condition medium has been shown to contain a variety of proteolytic enzymes, including serine and matrix metalloproteinases known to cleavage collagen.(22,23) Following incubation of the immobilized collagen with HUVEC-conditioned media, exposure of the cryptic epitopes were monitored by incubation with purified MAbs HUIV26 and HUI77. As shown in Figs. 4A and B, the cryptic epitopes defined by MAbs HUIV26 and HUI77 were specifically exposed in a time-dependent manner. In control experiments, serum-free conditioned media coated on plates in the absence of collagen showed no reactivity with these MAbs (data not shown). Importantly, both serine (Aprotinin) and matrix metalloproteinase inhibitors (EDTA) were shown to block the exposure of these cryptic epitopes, thus confirming a role for MMPs and serine proteases in the exposure of these unique sites (data not shown). The minimal reactivity observed with these antibodies to triple helical collagen in this assay was likely due to a small amount of thermal denaturation that may have occurred as a result of the time of incubation at 37°C. These findings provide further evidence for the cryptic nature of the HUIV26 and HUI77 epitopes and furthermore, indicate that these sites can be exposed by either proteolysis or thermal denaturation. XU ET AL. Exposure of the HUIV26 and HUI77 cryptic epitopes within human tumor tissues Our studies indicate that MAbs HUIV26 and HUI77 can recognize cryptic epitopes of purified collagen that were specifically exposed following proteolysis and thermal denaturation. However, collagen in vivo does not exist in isolation, but is rather interconnected within complex networks of many ECM molecules. Therefore, to determine whether these unique cryptic epitopes are exposed during invasive human tumor growth in vivo, we examined malignant human melanoma tumors by immunofluorescence staining with MAbs HUIV26 and HUI77. Tissue sections were prepared from biopsies of metastatic human melanoma tumors. Because collagen type IV is primary expressed as a component of the basement membranes of epithelial sheets and blood vessels, tumor sections were co-stained with a polyclonal antibody directed to factor VIII related antigen to mark the tumor blood vessels and with MAb HUIV26. MAb HUI77 recognizes cryptic epitopes present in a variety of collagen molecules, including collagen type I, which is expressed in the interstitial matrix surround the tumor cells. Thus, visualize the cellular components of these tumors, melanomas were co-stained with a polyclonal antibody directed to the av integrin subunit, which is known to be highly expressed within metastatic melanomas cells, and with MAb HUI77. As shown in Fig. 5A, the cryptic HUIV26 epitope (Green) was detected within human metastatic melanoma tumors. Importantly, the HUIV26 epitope appear to be primarily associated with the basement membrane of tumor-associated blood vessels. In contrast, the cryptic HUI77 epitopes (Green) were observed within the interstitial matrix surrounding tumor nodules, as well as surrounding many individual tumor cells (Fig. 5B). Interestingly, little if any reactivity was detected with these MAbs in frozen sections or normal human skin (data not shown). Taken together, these findings provide evidence for the exposure of unique cryptic collagen sites within human malignant melanoma. Moreover, our results also suggest that during human melanoma tumor growth in vivo, the three-dimensional structure of collagen is altered allowing exposure of unique sites defined by MAbs HUIV26 and HUI77. Further studies are know underway to more precisely identify the exact amino acid sequence of these cryptic epitopes and to determine if exposure of these cryptic collagen epitopes play a functional role in human melanoma tumor growth. DISCUSSION Collagen, the most abundant protein expressed in the body has been shown to play critical roles as a mechanical scaffold as well as regulating tissue morphogenesis and differentiation.(6–8) In fact, the crucial functions of collagen in biological systems can be demonstrated by the fact that a variety of human diseases are characterized by genetic defects in genes coding for collagen, such as Osteogenesis Imperfecta, Epidermolysis Bullosa, and Alports Syndrome.(24) Thus, an in-depth understanding of the cellular and biochemical functions of collagen on physiological processes is of great importance. While numerous studies have demonstrated the functional importance of intact triple helical collagen on biological processes, recent studies have indicated that collagen remodeling plays a critical GENERATION OF MAbs TO CRYPTIC SITES IN COLLAGEN 381 FIG. 3. ELISA analysis of MAb reactivity to genetically distinct forms of collagen. Microtiter wells were coated with 25 mg/mL of triple helical or thermally denatured purified collagen including, types I, II, III, IV, and V. Purified MAb antibodies HUIV26 (A) and HUI77 (B) were added to the wells (1.0 mg/mL) in a total volume of 50 mL and allowed to incubate for 1 h. The wells were washed and incubated with peroxidase labeled goat anti-mouse secondary antibody. Immunoreactivity was detected following addition of OPD substrate by measuring the optical density (O.D.) at a length of 490 nm. All data were corrected for nonspecific reactivity with secondary antibody. Data bars represent the mean O.D. 6 standard deviation from triplicate wells. role in a number of pathological events including, tumor growth and metastasis, arthritis, and diabetic retinopathy.(8,25,26) However, the cellular and biochemical mechanisms by which collagen remodeling contributes to these diseases is not completely understood. This gap in our knowledge is due in part to the lack of reagents that can specifically detect proteolytically remodeled collagen. Recently, some success has been achieved in the generation of antibodies that can recognize denatured forms of collagen type II.(27–30) These antibodies were developed by immunizing 382 XU ET AL. FIG. 4. MAbs HUIV26 and HUI77 react with cryptic sites within collagen following proteolysis. Microtiter wells were coated with 25 mg/mL of triple helical collagen types IV. Concentrated (203) serum-free-conditioned medium from HUVEC was added to the wells (100 mL) and allowed to incubate for 1, 6, and 24 h at 37°C. The wells were extensively washed with PBS/EDTA and blocked with BSA to prevent nonspecific binding. Purified MAb antibodies HUIV26 (A) and HUI77 (B) were added to the wells (1.0 mg/mL) in a total volume of 50 mL and allowed to incubate for 1 h. The wells were washed and incubated with peroxidase labeled goat anti-mouse secondary antibody. Immunoreactivity was detected following addition of OPD substrate by measuring the optical density (O.D.) at a length of 490 nm. All data were corrected for nonspecific reactivity with secondary antibody. Data bars represent the mean O.D. 6 standard deviation from triplicate wells. GENERATION OF MAbs TO CRYPTIC SITES IN COLLAGEN 383 FIG. 5. Detection of cryptic sites defined by MAbs HUIV26 and HUI77 within invasive human melanoma tumors. Biopsies of human metastatic melanoma tumors were embedded in OCT, snap frozen, and 4-mm-thick cryosections were prepared. Tumor sections were blocked with 2.5% BSA in PBS to prevent nonspecific binding. (A) Tumor sections were co-stained with MAb HUIV26 and a polyclonal antibody directed to factor VIII related antigen. Red indicates human blood vessels and green indicates exposure of the HUIV26 cryptic epitope. Yellow represents co-localization. (B) Tumor sections were co-stained with MAb HUI77 and a polyclonal antibody directed to the av integrin subunit. Red indicates cellular expression of av integrin and green indicates exposure of the HUI77 cryptic epitope. Yellow indicates co-localization. (Photomicrographs were taken at a magnification of 2003.) animals with isolated peptides or cleavage fragments of collagen.(27–30) These antibodies were used to detect soluble fragments of collagen from body fluids and tissue extracts.(27–30) Moreover, these antibodies were able to detect denatured collagen type II within osteoarthritis and rheumatoid arthritic tissues in vivo.(27–30) These findings suggest that degradation of collagen type II may play an important role in the pathogenesis of arthritis. In other studies, antibodies were developed against the propeptide domains of collagen type I. These antibodies have been used to detect soluble propeptides in serum and urine.(31–33) Interestingly, the levels of these soluble peptides have been shown to correlate with inflammation, hypertensive heart disease, and tumor metastasis.(32,34) These antipropeptide domain antibodies, however, do not detect degradation of triple helical collagen because these propeptides are cleaved as a normal process of collagen fibril formation and thus are more indicative of collagen maturation.(31–33) Thus, few antibodies exist that specifically react with proteoloyzed collagen types-I and IV. Therefore, highly specific antibodies to proteolyzed forms of collagen could have great utility not only in disease diagnosis and prognosis, but also in the study of the biochemical and molecular mechanisms contributing to cellular invasion. In this regard, SI was used to develop a set of MAbs to cryptic collagen epitopes. Similar SI protocols has been used successfully to develop MAbs directed to rare and low abundant epitopes.(35–38) In fact, SI has been used to generate specific MAbs directed to a variety of antigens, including sheep red blood cells and metastasis-associated antigens expressed on the surface of human tumor cells.(18,35–38) However, to our knowl- edge, this immunological approach has never been used to generate specific MAbs that recognize a conformationally dependent epitope within complex macromolecules such as collagen. In this report, we describe the use of SI to generate two MAbs (HUIV26 and HUI77) that recognize cryptic epitopes that are normally hidden within the three-dimensional structure of collagen. The epitopes defined by these unique MAbs are only exposed following thermal denaturation or proteolysis. While MAb HUIV26 is highly specific for a cryptic epitope within collagen type IV, MAb HUI77 recognizes a cryptic epitope that appears common within a number of genetically distinct forms of collagen including collagens types I, II, III, IV, and V. These MAbs can detect their respective epitopes in solid-phase ELISAs and Western blotting. Moreover, the cryptic epitopes defined by MAbs HUIV26 and HUI77 were exposed in invasive human melanoma tumors. Interestingly, the cryptic HUIV26 epitope was expressed primarily within the basement membrane of tumor-associated blood vessels and was not detected within the interstitial matrix. In contrast, the HUI77 cryptic epitope exhibited a more broad distribution pattern, showing expression mainly within the interstitial matrix and surrounding individual tumor cells. These findings are in agreement with the known distribution patterns of basement membrane collagen type IV and the various forms of interstitial collagen.(6–8) Our findings of exposure of distinct cryptic epitopes within malignant tumor with little if any detected in normal tissues, is consistent with the notion that increased proteolytic remodeling occurs during invasive tumor growth.(9–13) Our results raise many important questions such as, whether exposure of these unique cryptic sites are restricted to sites of melanoma 384 XU ET AL. invasion or whether they are also exposed in other diseased tissues? Does exposure of these cryptic sites correlate with a poor clinical prognosis? Moreover, do these cryptic collagen sites play a functional role in disease processes or are they simply a result of enhanced proteolytic activity? We are now in the process of performing experiments to answer these important questions. Taken together, our findings indicate that the SI protocol can be used to generate unique immunological reagents to conformational and cryptic epitopes of complex ECM proteins such as collagen. More importantly, our studies have generated a novel set of MAbs with unique binding properties that can be used to study the roles of collagen remodeling in human diseases. ACKNOWLEDGMENTS P.C. Brooks was supported by NIH grants CA74132-01 and ROI CA086140-01. The authors would like to thank Dana Jones for her expert technical assistance and Kathryn Carner for her help in the preparation of this manuscript. 16. 17. 18. 19. 20. 21. 22. 23. REFERENCES 1. Timpl R: Structure and biological activity of basement membrane proteins. Eur J Biochem 1989;180:487–502. 2. Zetter BR, and Brightman SE: Cell motility and the extracellular matrix. Curr Opin Cell Biol 1990;2:850–856. 3. Assoian RK, and Marcantonio EE: The extracellular matrix as a cell cycle control element in atherosclerosis and restenosis. J Clin Invest 1996;11:2436–2439. 4. Adams JC, and Watt FM: Regulation of development and differentiation by the extracellular matrix. Development 1993;117: 1183–1198. 5. Juliano RL, and Haskill S: Signal transduction from the extracellular matrix. J Cell Biol 1993;120:577–585. 6. van Der Rest M, and Garrone R: Collagen family of proteins. FASEB J 1991;5:2814–2823. 7. Prockop DJ, and Kivirikko KI: Collagens: molecular biology, diseases, and potentials for therapy. Ann Rev Biochem 1995;64:403– 434. 8. Olsen BR: New insights into the function of collagens from genetic analysis. Curr Opin Cell Biol 1995;7:720–727. 9. Chambers AF, and Matrisian LM: Changing views of the matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997;89:1260– 1270. 10. Mignatti P, and Rifkin DB: Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 1993;73:161–185. 11. Liotta LA, Steeg PS, and Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991;64:327–336. 12. Hiraoka N, Allen E, Apel IJ, Gyetko MR, and Weiss SJ: Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 1998;95:365–377. 13. Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aims RT, Stetler-Stevenson WG, Quigley JP, and Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin avb3. Cell 1996:85:683–693. 14. Timpl R, Wick G, and Gay S: Antibodies to distinct types of collagen and procollagens and their applications in immunohistology. J Immunol Methods 1977;18:165–182. 15. Scheinman JI, and Tsai C: Monoclonal antibody to type-IV colla- 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. gen with selective basement membrane localization. Lab Invest 1984;50:101–112. Monteagudo C, Merino MJ, San-Jaun J, and Stetler-Stevenson WG: Immunohistochemical distribution of type-IV collagenase in normal, benign, and malignant breast tissue. Am J Pathol 1990;136:585–592. Williams CV, Stechmann CL, and McLoon SC: Subtractive immunization techniques for the production of monoclonal antibodies to rare antigens. BioTechniques 1992;12:842–847. Brooks PC, Lin J, French DL, and Quigley JP: Subtractive immunization yields monoclonal antibodies that specifically inhibit metastasis. J Cell Biol 1993;122:1351–1359. Brooks PC, Silleti S, von Schalscha TL, Friedlander M, and Cheresh DA: Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 1998;92:391–400. Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, and Cheresh DA: Antiintegrin avb3 human breast cancer growth and angiogenesis in human skin. J Clin Invest 1995;96:1815–1822. Galfre G, and Milstein C: Preparation of monoclonal antibodies: Strategies and procedures. Method Enzymol 1981;73:3–46. Schnaper WH, Grant DS, Stetler-Stevenson WG, Friedman R, D’orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, et al.: J Cell Physiol 1993;156:235–246. Pepper MS, and Montesano R: Proteolytic balance and capillary morphogenesis. Cell Diff Dev 1990;32:319–328. Paepe A: The ehlers-danlos syndrome: a heritable collagen disorder as cause of bleeding. Thromb Haemost 1996;75:379–386. Petitclerc E, Stromblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AMP, Cheresh DA, and Brooks PC: Integrin avb3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res 1999;59:2724–2730. Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Hudson BG, and Brooks PC: New functions for noncollagenous domains of human collagen type-IV: Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem 2000;275:8051–8061. Srinivas GR, Barrach HJ, and Chichester CO: Quantitative immunoassays for type-II collagen and its cyanogen bromide peptides. J Immunol Methods 1993;259:53–62. Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, and Poole R: Damage to type-II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest 1995;96:2859–2869. Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, et al. Enhanced cleavage of type-II collagen by collagenase in osteoarthritis articular cartilage. J Clin Invest 1999;99:1534–1549, Otterness IG, Downs JT, Lane C, Bliven ML, Stukenbrok H, Scampoli DN, Milici AJ, Mezes PS: Detection of collagenase-induced damage of collagen by 9A4, a monoclonal C-terminal neoepitope antibody. Matrix Biol 1999;18:331–341. Fleischmajer R, Perlish JS, and Olsen BR: The carboxylpropeptide of type-I procollagen in skin fibrillogenesis. J Invest Dermatol 1987;89:212–215. Miyahara M, Njieha FK, and Prockop DJ: Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J Biol Chem 1982;257:8442–8448. Jukkola A, Tahtela R, Tholix E, Vuorinen K, Blanco G, Risteli L, and Risteli J: Aggressive breast cancer leads to discrepant serum levels of the type-I procollagen propeptides PINP and PICP. Cancer Res 1997;57:5517–5520. Diez J, and Laviades C: Monitoring fibrillar collagen turnover in hypertensive heart disease. Cardiovasc Res 1997;35:202–205. GENERATION OF MAbs TO CRYPTIC SITES IN COLLAGEN 35. Prigozhina TB, and Fontalin LN: Mechanisms of antigen-induced blockade of immune response and cyclophosphamide-promoted tolerance to Salmonella typhi Vi antigen. Eur J Immunol 1980;10: 641–646. 36. Many A, and Schwartz RS: On the mechanism of immunological tolerance in cyclophosphamide-treated mice. Clin Exp Immunol 1970;6:87–99. 37. Ker-hwa S, McDonald C, and Patterson PH: Comparison of two techniques for targeting the production of monoclonal antibodies against particular antigens. J Immunol Methods 1991;145:111–118. 38. Matthew WD, and Sandrock AW: Cyclophosphamide treatment used to manipulate the immune response for the production of mono-clonal antibodies. J Immunol Methods 1987;100: 73–82. 385 Address reprint requests to: Peter C. Brooks New York University School of Medicine Kaplan Cancer Center Department of Radiation Oncology and Cell Biology Rusk Building Rm 812 400 East 34 Street New York, NY 10016 E-mail: [email protected] Received for publication June 12, 2000. Accepted for publication July 13, 2000.