* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Membrane Adsorbers as a Tool for Rapid
Comparative genomic hybridization wikipedia , lookup
Maurice Wilkins wikipedia , lookup
Molecular evolution wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Western blot wikipedia , lookup
Cell membrane wikipedia , lookup
Endomembrane system wikipedia , lookup
Non-coding DNA wikipedia , lookup
Community fingerprinting wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
List of types of proteins wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Genomic library wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Molecular cloning wikipedia , lookup
DNA supercoil wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
DNA vaccination wikipedia , lookup
Deoxyribozyme wikipedia , lookup
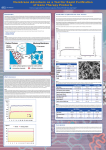
Membrane Adsorbers as a Tool for Rapid Purification of Gene Therapy Products P.R. Ball and I. Gyepi-Garbrah, Pall Life Sciences, Europa House, Portsmouth PO1 3PD, Hants, United Kingdom* Introduction Chromatography is a widely used tool for purification of biological products. Conventional chromatography matrices based on agarose and resin beads are generally well suited to the majority of applications for purifying biomolecules. Exceptions to this general rule are large molecules such as DNA and viruses. This is because of the small pore size of conventional chromatography matrices (typically of the order of 0.1 µm) which means that molecules enter these pores by diffusion. For large molecules, such as chromosomal or plasmid DNA or viruses, their very high molecular weight (up to several megadaltons) means that they enter the pores of conventional chromatography matrices very slowly (Figure 1). Pall Corporation has developed novel technology based on microporous polymeric membranes to overcome these limitations. Membranes have the advantage of large pores (up to 1 µm in size) that allow large molecules such as DNA and viruses to enter by convection, which is a much faster mass transfer process than diffusion (Figure 1). Because of their large pore size, membrane adsorbers can be operated at very much higher flow rates (up to 40 column volumes; CV/min) than conventional chromatography columns (which typically operate at under 5 CV/min). This allows much faster processing times. The technical challenge of using membranes as chromatography matrices has been that, when compared to conventional chromatography matrices, the larger pore size of membranes conferred on them a much lower internal pore area. This lower internal pore area meant that, historically, membranes had a much lower binding capacity per ml of matrix compared with conventional beads and resins. Novel technology has been used by Pall to give our membrane adsorbers the same capacity for small molecules (under about 300 kilodaltons) as conventional chromatography matrices and we describe here how this technology works. Three examples of how membrane adsorber technology can be used in processes relevant to Gene Therapy are described in this poster. These are: Purification of plasmid and virus vectors During the production of plasmid DNA and virus vectors, Pall Mustang membrane adsorbers can be used for the purification of the vector. Figure 3 shows data for the purification of plasmid DNA using a Pall Mustang membrane adsorber. Analysis of the purified plasmid DNA (Figure 4) demonstrated that > 90% of the purified plasmid DNA was in the desired supercoiled form. The purified plasmid contained < 0.1% protein and RNA, < 0.5% host chromosomal DNA and < 5 endotoxin units of endotoxin/mg DNA. The yield of plasmid DNA was 65-80% (Figure 4) Figure 5 shows data for the purification of a recombinant adeno-associated virus vector (rAAV). In the first step, a Pall Mustang S membrane adsorber was equilibrated with 1M NaOH,1M NaCl and loading buffer (3.33 mM MES/Hepes/NaOAc pH 6.4). Clarified cell lysate dilutred 1:3 with buffer was filtered through a Pall Mustang S membrane adsorber and unbound material was collected. This collected material was processed through a Pall Mustang Q membrane adsorber. rAAV-5 was eluted with buffer containing increasing concentration of KCl (100-1000 mM). Figure 6 shows comparative data for purifcation of Lentivirus using either gel filtration or Pall a Pall Mustang Q membrane adsorber Figure 3 Light adsorbance at 260 nm (A260: purple line) and conductivity (blue line) during purification of E. coli plasmid DNA from a 35 L volume of lysate using a 60 mL bed volume Pall Mustang Q membrane adsorber. The bed volume used was about 1/10 that of a conventional chromatography column yet the flow rate used was about 10 times that of a typical conventional chromatography column. (1) Removal of chromosomal DNA. (2) Purification of plasmid DNA (3) Purification of virus vectors Figure 1 Diagrammatic representation of the structure of a typical bead or resin particle used as the matrix in a conventional chromatography column (left) and of the membrane in a Pall Mustang™ membrane adsorber (right). The entry of molecules of the order of size of proteins and nucleic acids into the pores of a conventional chromatography matrix is by diffusion, whereas for a Pall Mustang membrane adsorber, it is by convection. Since convection is a much faster mass transfer process than diffusion, entry of large macromolecules is much faster into the pore structure of Pall Mustang membrane adsorbers than it is for conventional chromatography matrices. Figure 4 Data showing the purity of the plasmid DNA obtained as described in Figure 3. DNA clearance DNA is an important contaminant of processes that use mammalian cells e.g. expression of monoclonal antibodies. In the Food and Drug Administration (FDA) document ‘Guidance for Industry for the Submission of Chemistry, Manufacturing, and Controls Information for a Therapeutic Reconbinant DNA-Derived Product or a Monoclonal Antibody Product for In Vivo Use’ (Center for Biologics Evaluation and Research (CBER) Center for Drug Evaluation and Research (CDER) August 1996), the recommendation made for process validation is ‘A description and documentation of the purification process to demonstrate adequate removal of extraneous substances such as chemicals used for purification, column contaminants, endotoxin, antibiotics, residual host cell proteins, DNA and viruses, where appropriate should be proved’. Pall Mustang membrane adsorbers made using quaternary ammonium, or ‘Q’, chemistry have a high affinity for negatively-charged molecules, such as DNA, viruses, endotoxin and many proteins when these are processed at commonly-used pH values DNA fragments greater than about 2 kilobases in size are considered potentially carcinogenic (Handbook of Process Chromatography, 1997) and shouldtherefore be removed. Based on this, FDA CBER recommend in their document ‘Points to Consider in the Manufacture of Monoclonal Antibodies (1986) that "…it is suggested that, wherever possible, the final product should contain no more than 100 pg cellular DNA per dose…”. The data shown in Figure 2 demonstrates the ability of Pall Mustang membrane adsorbers based on ‘Q’ chemistry to remove genomic DNA from a solution of immunoglobulin G. Figure 2 The ability of a Pall Mustang Q membrane adsorber with a bed volume of 0.35 mL to remove genomic DNA from a solution of immunoglobulin G (IgG) was assessed using P32 labelled calf thymus DNA. A Pall Mustang Q membrane adsorber was challenged with a solution containing calf thymus DNA (19.85 ug/ml total concentration) and bovine IgG (1.65 mg/ml) in phosphate-buffered saline containing 150 mM NaCl. The flow rate used was 20 CV/min. The light adsorbance of the effluent from the device tested was monitored at 260 nm and 280 nm. Fractions (14 mL) were collected and the presence of radioactively-labelled calf thymus DNA in fractions 1, 2, 3, 4, 5, 10, 15, 20, 21, 23, 24 and 25 were analysed using a hybridisation-based dot blot method. Figure 2a shows the adsorbance at 260 nm and 280 nm during this experiment and Figure 2b the level of calf thymus DNA in the solution tested before (purple) and after the device evaluated. No DNA was detected in the filtrate until the device was saturated and breakthrough of DNA was detected (shown by the upward inflection in the red line to the right of the figure). The capacity of the device was measured at 6.4 mg, or 18 mg/mL DNA capacity. The light adsorption traces show that no detectable removal of IgG occurred. Figure 2a Criteria Results A260/280 >1.75 to <1.95 SC Form >90% Endotoxin <5 E.U./mg DNA Protein <0.1% RNA <0.1% E. coli DNA <0.5% Yield 65% - 80% Figure 5 Data for purification of rAAV-5 using Pall Mustang S followed by Pall Mustang Q membrane adsorbers in comparison to caesium chloride density gradient centrifugation. OPU = optical particle units, TCID50% = 50% tissue culture infective dose, i.e. dilution of virus suspension tha ‘kills’ 50% of cell cultures Crude Preparation Mustang Q 12.5 mL (starting) 3.5 mL 3 mL 5 x 108 of 293S cells 2.5 x 108 of 293S cells 2.5 x 108 of 293S cells Total infectious particles (TCID50%) 5.2 x 1012 1.15 x 1010 1.98 x 1010 Infectious particles/mL (TCID50%/mL) 4.16 x 1011 3.3 x 109 6.6 x 109 N/A 3.65 x 1012 3.85 x 1011 Total Optical Density (OPU) N/A 1.27 x 1013 1.15 x 1012 Total protein (mg) 830 82.31 14.38 Protein (mg/mL) 66.4 23.51 4.79 6.26 x 109 1.39 x 108 1.38 x 109 Total volume Number of infected cells for each method Optical Density (OPU/mL) Specific Activity (TCID50%/mg) CsCI2 Method Figure 6 Purification of Lentivirus using a Pall Mustang Q membrane adsorber. A 60 mL bed volume Pall Mustang Q membrane adsorber was equilibrated with 1 L 150 mM NaCl, pH7.5. Material from the viral harvest was loaded at a flow rate of 200 mL/min. Wasing (400 mM NaCl) was followed by elution (using 1.5 M NaCl) and the eluate diluted with water to approximately 150 mM NaCl. Both methods achieved comparable vector purity, but the method using a Pall Mustang Q membrane adsorber is easier to scale up. Gel Filtration Finish Start Finish 3.16 x 107 3.29 x 108 1.09 x 107 2.17 x 108 Total protein (µg/mL) 4684 31.2 2202 169 P24 0.35 2.36 0.18 2.09 7.74 x 10-5 7.56 x 10-2 8.17 x 10-5 1.23 x 10-2 Titre (TU/ml) P24/Total protein Figure 2b Mustang Start Conclusions Pall Mustang membrane adsorbers provide a rapid, easy to use, scaleable technology for the purification of plasmid DNA and virus vectors and associated vaccines, which are important tools in gene therapy. In addition, Pall Mustang Q membrane adsorbers provide a simple, rapid, disposable technology for DNA clearance in processes where genomic DNA represents an undesired contaminant of the final product. In addition, unlike conventional chromatography matrices, Pall Mustang membrane adsorbers are supplied as disposable, ready-to-use modules with no column packing required, which makes them simple and convenient to use and avoids the need to validate cleaning prior to reuse which is often necessary with other chromatography systems. 2/04, GN04.xxxx ©2004, Pall Corporation. Pall, , and Mustang are trademarks of Pall Corporation.