* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mesenchymal Dysplasia: A Recessive Mutation
Genome evolution wikipedia , lookup
Public health genomics wikipedia , lookup
Population genetics wikipedia , lookup
Gene expression programming wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene expression profiling wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Genomic imprinting wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Oncogenomics wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Frameshift mutation wikipedia , lookup
Genome (book) wikipedia , lookup
Nutriepigenomics wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Microevolution wikipedia , lookup
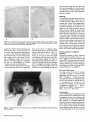
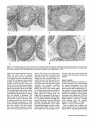
Mesenchymal Dysplasia: A Recessive Mutation on Chromosome 13 of the Mouse H. O. Sweet, R. T. Bronson, L. R. Donahue, and M. T. Davisson Mesenchymal dysplasia (mes) is a new autosomal recessive mouse mutation that alters normal growth of mesenchyme-derlved tissues and provides a new mouse model for studying connective tissue development and defects. Mutants are characterized by preaxial polydactyly of all four feet, a shortened face, wide set eyes, domed head, and a shortened kinky tail. Multiple skeletal defects are seen in alizarin-stained specimens. Hlstologlcally, areas of mineralization are found In tendons. Mutants also have increased musculature In the shoulders and hips and decreased peritoneal fat. Salivary glands, testes, and kidneys are smaller than in llttermates. Mesenchymal dysplasia has been mapped to mouse chromosome (Chr) 13. These mapping crosses also confirmed that the Purklnje cell degeneration (pcd) mutation is on Chr 13. From The Jackson Laboratory, 600 Main St., Bar Harbor, Maine 04609-1500 (Sweet, Bronson, Donahue, and Davisson) and Tufts University, School of Veterinary Medicine, Boston, Massachusetts (Bronson). We dedicate this paper to Dr. Margaret C. Green. This work was supported by grants BIR 89-15728 from the National Science Foundation, P40 RR01183 and Cancer Core Center grant CA34196 from the National Institutes of Health, and a gift from the Eleanor Naylor Dana Charitable Trust. The antlserom (UB2-495) to IGF-1 was a gift from the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Child Health and Human Development, and the U.S. Department of Agriculture. We thank Belinda Harris, Ann Hlgglns, and Prlscllla Jewett for hlstologlcal preparations, W. a Beamer lor helpful discussions, Unda Nelesld for manuscript preparation, and Drs. Margaret C. Green and Kevin Flurkey for critical review of the manuscript. Address reprint requests to H. 0. Sweet at the address above. Journal of Heredity 1996^7^7-95; 0022-1503/96/J5.00 Inherited malformation syndromes in mice provide valuable model systems for understanding gene defects creating similar human conditions (Winter 1988). With the rapidly expanding mouse genetic map, the structural genes for many mutations causing such syndromes are now being identified. Thus, each new mouse mutation that causes congenital malformations provides the potential of identifying the gene affected in comparable human conditions. In addition, mouse mutations that cause developmental abnormalities provide model systems to study basic mammalian development. We describe here a new recessive mutation in the mouse that affects many mesenchyme-derived tissues. The mesenchymal dysplasia(mes) mutation causes multiple skeletal anomalies, including preaxial polydactyly of all four feet, a shortened and wider than normal face with wide set eyes, a domed head, a broad thoracic region, and a kinky tail. The most striking features of the mutant are excess skin and increased musculature in shoulder and hip regions. Failure of the testes to descend results in sterility in virtually all males. Mesenchymal dysplasia maps to mouse chromosome (Chr) 13 near four genes that involve growth regulation. Materials and Methods Mice All mice were reared and all genetic breeding studies were carried out in the Mouse Mutant Resource at The Jackson Laboratory (Davisson 1990). Mice were maintained in a modified barrier mouseroom. The room with a filtered air supply is maintained at 68°F, 40-50% humidity. Mice are caged in polycarbonate cages (51 in2) on sterilized white pine shavings. All boxes are covered with a flat filter. Water supplied to the animals is both acidified and chlorinated with a pH of about 2.5, residual chlorine content of 12 to 18 ppm. The water treatment program suppresses the growth and spread of Pseudomonas sp. The strain is maintained on the 96W diet formulation manufactured by Emory Morse Company, Guilford, Connecticut (protein 22.48%, fat 7.13%, fiber 2.58%, ash 4.57%, Ca 0.22%, P 0.67%). Descriptions of the mutant genes that were used in tests for allelism may be found in Green (1989) or in MGD (1994). Genetic crosses are described in detail in Results. Phenotypic Studies Eleven pairs (four female pairs, seven male pairs) of mes/mes and littermate controls (+/?) were weighed at weekly intervals (to the nearest 0.1 g) for a 4 week period. The sample included three different groups of mice starting at 3, 5, and 11 weeks of age, respectively. Seven of these pairs (three female, four male) and two known heterozygotes (+lmes) were euthanized by CO2 and necropsied. Their organs were weighed to the nearest 0.1 mg, tissues saved for histology, skins mea- 87 sured, and carcasses saved for skeletal preparations. Skins were measured by laying them flat on paper towels and measuring length and width in millimeters using a standard metric ruler. Alizarin-stained whole skeleton preparations were made using the method described by Green (1952). Histology Tissues for most histological examinations were fixed in formal-acid-alcohol or Bouln's solution. Bones were demineralized for several weeks in Bouin's solution. Representative paraffin sections of all tissues from six adult mes/mes mice were stained with hematoxylin and eosin (H&E). Multiple sections of brain from one mutant adult were stained with H&E; replicate brain sections were stained with luxol fast blue and cresylecht violet. For histological examination of testicular tissue, testes were fixed for 1 h in 2% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate buffer, embedded in JB4 resin, sectioned at 1 mm, and stained with H&E for general examination and with periodic acid-Schiff (PAS) for analysis of acrosome development. Histological sections were examined from testes of two 1 month old and two 2 month old pairs of mes/mes mutants and littermate +/? controls. Body Composition Methodology The amounts of water, fat, protein, and mineral that compose the body mass were measured using a biochemical protocol previously described (Donahue and Beamer 1993). Briefly, four mutant and three normal +/? mice 8 weeks of age were fasted overnight and blood samples were collected by retro-orbital bleeding. Mice were euthanized by exposure to CO2 and then shaved of all hair. Gastrointestinal tracts were emptied of food contents, carcasses weighed, tail lengths measured, and mice were stored frozen at -4°C until analyzed. Deep frozen (liquid Nj) carcasses were pulverized, lyophilized, and dry weights obtained. Body water data were derived from the differences between fresh carcass and lyophilized weights. Entire lyophilized samples were homogenized to uniform consistency and aliquots repeatedly extracted with chloroform:methanol (3:2, v/v) to remove lipid; the defatted pellet was weighed. The difference between lyophilized mass and defatted pellet was the amount of fat. The defatted pellet was ashed overnight at 600°C and the ash weighed to determine the amount of min- 8 8 The Journal oi Herecfity 1996:87(2) eral. The difference between the defatted pellet and ash weight yielded the amount of protein. These data were corrected for aliquot size to obtain the final absolute amounts of fat, protein, and mineral present in each carcass. The data are expressed as a percent of total carcass weight and were analyzed without arcsine transformation because the range of percentages within a given data set was less than 40 (Little and Hills 1975). first analyzed for main effects using ANOVA. If significant main effects were identified, individual means were compared by Fisher's least significant difference (LSD) test or by Student's t test. Differences were considered significant at p < .05. Recombination estimates were calculated using a computer program for intercross data (Green 1985). Hormone Measurements Serum insulinlike growth factor I (IGF-1) levels in seven mes/mes and six +/? littermates (genders combined) were analyzed by radioimmunoassay (R1A). The R1A is composed of (1) antibody (UB3-189) produced by Underwood and Van Wyk and obtained from the National Hormone and Pituitary Hormone Program; (2) recombinant human IGF-I (rhIGF-I) from Bachem (Torrance, California); and (3) 12S1-IGF-I from Amersham (Arlington Heights, Illinois). Serum samples were prepared for R1A of IGF-I by extraction with acid-ethanol followed by neutralization (Donahue and Beamer 1993). Serum IGF binding proteins (IGFBPs) were measured by Western Ligand Blotting as described previously (Donahue et al. 1990). Briefly, sodium dodecyl sulfatepolyacrylamide gel electrophoresis was performed according to Laemmli (1970) on serum proteins separated under nonreducing conditions in 12.5% acrylamide gels at constant current (36 mA) for 6 h. The separated proteins were transferred to nitrocellulose using the method of Towbin et al. (1979) and then washed in 3% NP40, blocked with 1% BSA in TBS, and rewashed in 0.1% Tween. Electroblotted proteins were incubated for 24 h at 4°C with 200,000 cpm 12SI-IGF-i and then exposed to Kodak XOMAT AR film for 4-6 days at -70°C. Radiolabeled IGFBPs on the autoradiograms were confirmed by l4 C-labeled molecular weight standards (Sigma, St. Louis, Missouri) and then graphed with a CliniScan 2 Scanning Densitometer (Helena Laboratories, Texas). To uniformly quantify the IGFBPs, paper densitometric peaks were cut out and weighed. The amount of each IGFBP present in sera of mes/mes and +/? mice was expressed in mass units for statistical evaluation of differences between genotypes. Origin and Description Mesenchymal dysplasia arose in and was originally maintained on the inbred CBA/J background (Sweet and Bronson 1988). Reproductive performance of the CBA/J strain (Les 1991) made it difficult to continue inbreeding or to maintain this mutation on the CBA/J background. Therefore, the original heterozygous +/mes male parent was outcrossed to a B6C3Fea/a (C57BL/6JLe x C3HeB/FeJLe-a/a) hybrid female. Having this mutation on the B6C3Fe-a/a hybrid background has several advantages: it permits use of the technique of ovarian transplantation for ease of maintenance; litter size is increased, reducing the risk of losing the mutation, and the homozygotes produced are hardier and live a normal life span. The mutation is maintained in the Mouse Mutant Resource by repeated backcrossing to the B6C3Fe-a/a hybrid background. Mice from both backgrounds were used to characterize the phenotypic effects of the mutation; they did not differ significantly. Statistical Analysis Descriptive statistics were computed for all data by genotype and sex. Data were Results The first mutants submitted to the Deviant Search Program were recognized at weaning age by preaxial polydactyly of all four feet, dome-shaped head, white belly spot, and kinky tail. The parents of this litter, a sibling pair of CBA/J, produced a total of 13 progeny in three litters, of which four abnormal and seven normal offspring were classified. The growth of the affected animals was retarded compared to normal siblings and most died before 40 days of age. On both the original background and the present B6C3Fe stock, the mes/mes phenotype is characterized at birth by preaxial polydactyly of all four feet, a shortened face, wide set eyes, domed head, and kinky tail. The overall body configuration of the mutant between birth and weaning is shorter than that of the normal sibling. The thoracic region is broader than normal. Despite the shorter body, internal viscera are accommodated within the body cavities without causing a bulging of the sides such as is seen in homozygous brachymorphic (bm/bm) mice. Figure 1. Mutant mes/mes (right) compared to +/? littermate (left). Note folds of skin on the face and the apparent small size of mutant eyes because of the surrounding excess skin. The mutants shown are on the original CBA/J genetic background. A few severely affected homozygotes display kyphosls in the shoulder region. No scoliosis Is observed. The skull may have either a small frontal dent or slight bulge. Eyes are wide set and flattened rather than bulging. The entire head appears foreshortened and wider (see Figure 1). There are no externally visible mandibular defects, cleft lip, or cleft palate. Rear legs are adducted to the sides at right angles to the spinal column. Tails are thickened, shortened, and kinked. Mutants may have a white belly spot. By 5 to 6 weeks of age, the affected animal appears larger than its normal littermates. Folds of skin obscure the eyes and cover the joints of the legs. Overall, the skeletal frame of the mutant appears unable to accommodate the ex- Figure 2. Alizarin-stained skeletons of thorax of mes/mes (right) and +/? (left) llttermates. Ventral view showing abnormal sternum. Note that although the mutant thorax seems smaller it Is wider In proportion to length than the normal. Figure 3. Alizarin-stained skeletons of left hind foot of mes/mes (right) and +/? (left) littermates Illustrating extra preaxlal toe (left arrow) and extra bone spur (right area) abnormalities of the mes/mes mouse. cessive amount of skin. Although this results in the appearance of disproportionately shortened long bones in the extremities, alizarin preparations show normal long bone proportions. Homozygous mes/ mes adults have visibly thickened, tough foot pads. Most homozygotes fail to reproduce (see Reproduction section). The mes/mes homozygote is behaviorally different from its normal littermates in that it is extremely docile. Morphology Measurements of the alizarin-stained skeletons showed no differences in length or width of long bones between mes/mes and +/? controls. Caliper measurements of scapula, humerus, ulna, tibia, fibula, and skull length and width showed no significant differences between mutant and control mice (data not given). No overt hydrocephalus was seen in any of the mutants examined histologically, despite the slight doming of the skull visible externally. Although the width between the eye sockets in mes/mes adult mice was consistently greater than in controls, no extra interfrontal bone was found. The mean interocular width in eight mes/mes mutants was 1.00 cm while that of seven +/? adults was 0.65 cm. The most obvious skeletal abnormalities were seen in the thorax (Figure 2) and legs (Figure 3). All affected mes/mes mutants exhibited fusions of sternebrae and an abnormally shaped manubrium, Sweet et al • Mesenchymal Dysptasia 89 about 6 weeks of age. After 6 weeks of age the mutants weighed more than their normal littermates. Within individual litters the differences were not always significant (data not shown). Figure 4. Cross sections of sacral spine and epaxial musculature from (a) a wild-type mouse and (b) a mes/mes mouse (both 5 months old), photographed at the same magnification. Note the dramatic hyperplasla of muscle fibers In the mutant. Hematoxylln and eosln, magnification X12.7. usually two distinct bones which had not fused at the caput. In some mes/mes mutants ribs may be asymmetrically fused as a result of the abnormal xiphisternum. The overall result is a wider thoracic basket in relation to the whole body. The skeletal preparations made from the two known +/mes heterozygotes to determine the effect of heterozygosity on skeletal structure showed no abnormalities. Skins of the mes/mes mutant were found to be consistently wider and longer than those of the normal +/? sibling, confirming the impression that the living mutant seems to have excess skin (W = 8.6 ± 0.4 versus 6.0 ± 0.2; L = 11.3 ± 0.8 versus 10.8 ± 0.7; four pair of males). No significant differences in heart, liver, spleen, thymus, and adrenal weights were found in the limited sample examined to date (n = 4 pairs). Peritoneal body fat was lacking in all 11 homozygotes examined. In general, mutants of both sexes weighed less than their like-sex normal sibling controls until Histology The musculature of adult mutant mice was dramatically increased in mass due to increased numbers of fibers rather than increased size of fibers. Both appendicular and epaxial muscles were involved. The muscular hyperplasia of mes/mes mice is easily seen in cross sections of hip and lumbar muscles (Figure 4). Muscle fiber hyperplasia was more pronounced in male than in female mutant mice, but was present in both sexes. Histologic examination of achilles tendon of mutant mice revealed that the collagen was mineralized, but neither bone nor cartilage was present in the tendons. Other organs were without significant lesions. The sections of cerebrum and other areas of the brain of mutants had no lesions, which might have accounted for their unusual docile temperament. Biochemical Body Composition Analyses Biochemical analyses of entire carcasses showed that the mes/mes males (n = 4) have a higher percent body water (67.8% versus 63.4%), and lower percent body fat (22.6% versus 29.6%) than their normal littermates. Differences in percent body protein (8.6% versus 6.0%) and mineral (0.99% versus 0.86%) were not significant. IGF-I and IGFBP Levels The mean serum IGF-I level of mes/mes mice (486 ± 34 ng/ml, n=7) did not differ significantly from that of the +/? controls (540 ± 35 ng/ml, n = 6). We found, however, that the total serum binding capacity for IGF-I was reduced in mes/mes mice (n = 7) compared to the +/? mice (n = 5), and that the reduction was due primarily to significant reduction (p = .003) in IGFBP3 levels (IGFBP3 in mes/mes mice = 28.8 ± 3.1; in +/? = 54.8 ± 6.7). Figure 5. Ventral view of 3 month old mes/mei male on the B6C3Fe-a/a hybrid background. Illustrating abnormal testlcular position. 9 0 The Journal of Heredity 1996:87(2) Reproduction On the B6C3Fe genetic background both sexes of mutant mice seldom breed. A few homozygous females have become pregnant but failed to deliver viable offspring. Male mutants are probably infertile because the testes are usually undescended. Testes either remain in the abdominal cavity or come to rest outside the normal scrotal position under the skin of the upper rear legs (Figure 5). In some cases the Figure 6. Cross sections of testes from mes/mes mutant male and +/? age-matched llttermate. (a) tubule In abdominally located testis from mes/mes, note vacuolated cells and no sperm In lumen; (b) tubule from opposite testis of the same mes/mes male as (a), but located under skin of leg, note less severely affected; (c) +/? llttermate control; (d) tubule cross section from Xpl/Y male, testis located low In abdomen near Inguinal canal. All magnifications «• X1333 inguinal canal appeared blocked, while in others the testes could be manually pushed into the scrotal sac. Examination of the inguinal area of one mes/mes mouse revealed that the inguinal canal extended through the inguinal ring halfway to the location of the scrotal sac, if that were present. Since no scrotum had developed, the testes could descend into the inguinal region along the inner aspect of the thigh. The preputial glands and seminal vesicles are approximately one-fourth the size of control glands, suggesting that the mutant mice are deficient in androgens. This might account for the failure of the inguinal canal and scrotum to develop. Levels of gonadal steroids have not been measured. Histologic examination of testis sections showed that undescended testes had fewer tubules with open lumens. There were reduced numbers of mature sperm and few sperm tails evident in the lumens. The severity of the effect correlated with the position of the testis in the abdominal cavity. One completely internalized testis was composed mostly of interstitial cells and had only type A spermatogonial cells, with multinucleate syncytia. Sertoli cells and cells with clumped chromatin and vacuolated cytoplasm (suggesting degeneration) often were found in the center of the lumens (Figure 6a). Although testes positioned along the leg or undescended testes with open inguinal canals had maturing germ cells (Figure 6b), they appeared to be fewer in number than in littermate controls (Figure 6c). The mouse mutations X-linked polydactyly (Xpl) (Sweet and Lane 1980) and dominant hemimelia (DH) (Searle 1964) also cause cryptorchidism, and a similar histological phenotype was seen in undescended testes of Xpipi and Dh/+ mutant males (Figure 6d). Testis weight is less in mes/mes males than normal littermates, consistent with the reduction in germ cell numbers. Genetic Analysis Mesenchymal dysplasia is inherited as a recessive mutation. The mating between the original heterozygous +/mes male (sire of the first mutant mice seen in the Deviant Search) and the B6C3Fe-a/o F, female yielded all normal progeny. Matings among these F, progeny resulted in the appearance of homozygous mes/mes affected animals in the F2 generation. Of 324 offspring from known carriers, 58 were classified as mes/mes, a frequency not significantly different from the expected frequency of 0.25 (58/324 = 0.1790, x 2 = 3.2654, p > .07) for a recessive gene. Tests for allelism were negative with known mutations with one or more similar skeletal defects, including brachymorphic Sweet et aJ • Mesenchymal Dysplasia 91 Table 1. Two-point crosses between mes and genes on Chr 13 Phenotype of progeny Cross Female 1 2 mes +/+ bg mes +/+ mu 3 mes mu/+ + male ++ mes + + m* mes m Total RE ± SE X X mes +/+ bg mes +/+ mu 130 241 50 63 98 5 57 248 396 X mes mu/+ + 38 — 3 9 50 28.70 ± 5.75 17.3 upper 95% confidence level 6.59 ± 3.65 • m — marker bg or mu. (prri), brachypody (bp"), congenital hydrocephalus (c/?), flexed-tail (f), short-ear (se), and extra toes (Xt1). Linkage was first found with the recessive coat color mutation beige (bg) on proximal Chr 13, suggesting that mes was probably located toward the distal end of Chr 13 (recombination = 28.74 ± 5.70) (Table 1, cross 1). Subsequent crosses with the more distal Chr 13 gene muted (mu), a second recessive coat color mutation, confirmed this location. Since each gene (mu, mes) is recognizable at birth, all offspring were classified at birth and again at weaning. No preweaning mortality and no recombinants between mu and mes (mice homozygous for both mutations) were observed among the 396 F2 progeny classified (Table 1, cross 2). The absence of double mutants may have been due to prenatal loss, although the observed litter size suggested such loss was not substantial. If there was prenatal loss in cross 2 or crosses 6 or 8, the loss does not appear to affect the calculation of recombination estimates, since these are internally consistent and consistent with previously published data for these Chr 13 genes. To determine if recombination had taken place, 16 mu+/mu? females from the F2 generation were progeny tested for mes by mating to heterozygous +/mes males. At least 12 offspring were raised from each female tested. Five of 16 females tested from this and subsequent crosses were found to carry the mes gene, giving a recombination estimate of 18.52 ± 8.14% calculated by the method given in Deol and Green (1966). A recombinant chromosome with mu and mes in coupling was recovered and used for a coupling intercross (Table 1, cross 3). To determine the exact position of mes on Chr 13, mice carrying mu and mes in coupling (from cross 3) were mated to homozygous pearl (pe) mice from the inbred C3HeB/FeJ-pe strain, and F,s mated in the three-point intercross shown in Table 2, cross 5. Homozygous +pef?pe or mu+pe/mu ?pe progeny from this cross were further tested by breeding for the presence of mes. Seven of the 17 individuals tested were found to carry the mes gene giving a recombination estimate between pe and mes of 25.93 ± 9.49% [calculated according to the method given in Deol and Green (1966)]. Two of the five mu homozygotes from this cross carried a recombinant chromosome with mu and mes. These data together with the data from cross 2 above gave a total of seven mice with recombination between mu and mes in a total of 21 tested for a recombination estimate of 20.00 + 7.41 (Deol and Green 1966). Further three-point intercrosses (Table 2, crosses 6 and 7) were constructed by appropriate matings from such tested individuals. Finally, a three-point intercross using a fourth Chr 13 marker gene, Purkinje cell degeneration (pcd), was done (Table 2, cross 8). A female homozygote, pcd pelpcd pe, obtained from a two point cross between B6C3Fe-a/a pcd X C3HeB/FeJ-pe F,s (Table 3, cross 4), was used as a donor for an ovarian transplant. The recipient female was then mated to a heterozygous +/mes male from the B6C3Fe-a/a-mes colony. F, progeny of genotype +pcd pe/?+ + , were mated together to test for the presence of mes. F2 progeny from matings producing mes/mesoffspring were scored at birth for mes, at 7 days for pe, and at weaning for pcd. The cross between pcd and pe shown in Table 3 was done to confirm that pcd maps to Chr 13. Combining the data from all crosses for each pair of loci gives the recombination estimates shown in Tables 4 and 5. Although each of the three-point crosses involving the mes gene failed to produce some of the expected progeny genotypes, the data taken together allow us to position mes near the middle of Chr 13. Based on the assumption that the least frequent pairs of complimentary recombinant classes is the double recombinant class we conclude that the most probable order is bg-mumes-pcd-pe (Figure 7). Cross 7 was omitted from the calculations for the mu-pe interval because the distance in this cross is significantly different from all other data for this interval (cross 6; Cattanach et al. 1994; Lyon and Meredith 1969). Dumpy (dpy), a mutant gene with skeletal effects located on Chr 13 "fairly close" to mu (Hollander 1981) has not been tested for allelism with mes and, indeed, may be extinct. Wakasugi et al. (1988) describe a mutation causing facial and jaw abnormalities that was induced by a transgenic insertion Into a Chr 13 gene. Although we did not test for allelism with mes, no facial or jaw malformations were seen in alizarin preparations of mes/ mes mutant mice. Discussion We have discovered and characterized a new autosomal recessive mutation in the mouse that causes malformations in many mesoderm-derlved structures. Because of the wide range of pleiotropy in affected mutants, it is likely that the basic defect occurs early in development. Thus, we have named the mutation mesenchymal dysplasia (mes). Some of the effects of mes are common manifestations of pleiotropy in mouse mutants of this type, including polydactylism, tail kinks, and belly spots. Skeletal defects, such as abnormalities of the xiphistemum, may be common but difficult to detect in live animals. Located on mouse Chr 13 are several somewhat simi- Table 2. Three-point crosses confirming linkage of mes with genes on Chr 13 Cross Female x mu ma +/++ pe mu + pe/+ mes + mu mes pe/+ + + mes ++/+ pcd pe 9 2 The Journal oi Heredity 199687(2) male Phenotype of progeny mu mes + + + pe mu + pe mu mes +/++ pe mu + pe/+ mes + 145 119 150 22 +++ mes + + 21 2 28 7 mes pcd pe mu mes mes ++/+ pcd pe 66 49 37 + pcd pe 32 + mes + 32 77 20 + pcd + mu mes pe mu ++ 11 39 43 mes + pe + mes pe Total 1 3 mes pcd + + + pe 261 301 223 Total 137 Table 5. Summary of all linkage data including combined data from all crosses involving the same genes Table 3. Two-point cross between pcd and pe on Chr 13 Phenotype of progeny Cross Female X male + + pcd + +pe pcdpe Total RE±SE 4 pcd +/+ pe X pcd +/+ pe 194 75 83 1 353 19.19 ± 3.99 lar pleiotropic mutations, including congenital hydrocephalus (ch), dumpy (dpy), flexed-tail (f), and the extra toes mutations in the GLHCruppel family member GLJ3 gene (GUV, GU3™, GliS*"*"). The effect of mes on the formation of the sternum is similar to that produced by the mouse mutations short ear (se) on Chr 9, ch, and Xt on Chr 13 (Johnson 1986). Of the Chr 13 mutations only dpy maps in the region of mes and we have been unable to test the two genes for allelism. For a brief description of these genes see Green (1989), and for a more complete analysis see Gruneberg (1952) or Johnson (1986). Of these mutations only Xt is thought to be homologous to a human condition (Greig cephalopolysyndactyly syndrome). The features unique to the mes mutation are the wide spaced eyes, the loose skin, the extremely thick tough footpads, and docility of the homozygote. The excess skin, wide face, hyperplasia of muscle, mineralization foci in tendons, and abnormal sternum structure suggest overproduction of a growth factor or growth factor-related product that affects mesodermderived structures. We have previously reported two other mutations that cause excess growth of mesoderm-derived tissues, but mes produces a phenotype distinctly different from either. Tight-skin (Tsk) causes excessive subcutaneous connective tissue that is caused by an increase in both cellularity and amount of intercellular material in the skin, and excess bone growth (Green et al. 1976). Tsk causes a defect in regulation of biosynthesis of three specific collagen molecules in dermal fibroblasts (Jimenez et al. 1986). The recessive mutation progressive ankylosis (ank) causes excess amounts of fibrous tissue, cartilage, and bone primarily around the joints. Like Tsk, ank causes an increase in cellular proliferation (Sweet and Green 1981). Excess tissue in the mes/mes mutant is primarily in the entire skin and in the muscle tissue and is due to hyperplasia rather than hypertrophy. Skeletal muscle hyperplasia is a striking pathologic feature of mes/mes mutants. It is similar to the bovine model of increased musculature, double-muscling (DM) (Ashmore 1974). The observations of increased musculature of adult mutants and the lack of peritoneal body fat led us to do body composition analyses of the mutant. Biochemical analyses, however, revealed that although body water was increased and body fat was reduced in mes/mes males, body protein and mineral were not significantly different from those of normal littermates. The lack of statistically significant differences in whole body composition may be because the increased muscle in mes/mes mutants is concentrated in shoulder and hip areas and is not uniform throughout the entire body. This is consistent with their reduced or unaffected organ weights, illustrating selectively increased protein accretion. The increased musculature is probably not androgen driven. If this were the case one would ex- Interval Crosses RE + SE mej-og mes-mu mes-pcd mes-pe mu-pe pcd-pe 1 2,-3 ,5 , 6 , - 7 8 5,6, 7, 8 5,6 4,8 28.7 ± 5.8 17.2 ± 1.8 S29.1 12.7 ± 2.1 33.70 ± 2.8 15.4 ± 25 Data were combined using the average weighted method of Mather (1946). • Recombination estimate from tested mice in crosses 2 and 6 used to calculate combined values. pect androgen sensitive organs (salivary glands, testis, and kidneys) to be enlarged, whereas the mes/mes males have reduced salivary glands, testes, and kidney mass. Serum androgen measurements will be needed to confirm this observation. Since IGF-I has been shown to induce increased muscle protein synthesis (Pell and Bates 1992; Tomas et al. 1991), we considered the IGF-I/IGFBP axis as a possible mechanism for the increase in muscle protein accretion in mes/mes mice. IGFBP3 is the largest of the IGFBPs and serves to both transport IGF-I in circulation and to prolong its half-life (Baxter and Martin 1989). While IGFBP3 in mes/mes mice was reduced to approximately half that of control mice, serum levels of IGF-I in mes/mes mice were not different. An increase in IGF-I synthesis could account for normal serum levels of IGF-I despite the decreased serum IGFBP3 and potentiate the biological action of IGF-I in skeletal muscle tissue by allowing greater quantities of this growth factor to reach its target tissue. To establish the role of IGF-I/ IGFBP3 in this mutant, we plan further studies to measure IGF-I and IGFBP3 synthetic rates, and to examine the response T Table 4. Recombination estimates for three-point crosses in Table 2 calculated by combining like crosses F, genotype Linkage with mes: mes +/+ mu mes mu/+ + mes +/+ pcd mes +/+ pe mes pe/+ + Linkage among other loci: mu +/+ pe mu pe/+ + Crosses mes + + nr mes m Total RE ± SE 13.07 upper 95% confidence level 17.07 ± 1.82 29.1 upper 95% confidence level 12.95 ±3.71 12.59 ± 2.40 28.7 ±5.8 2,6 3,5,7 8 5,6,8 7 397 382 74 389 150 135 55 22 152 20 165 23 41 156 7 74 — 2 46 697 534 137 699 223 mupel+ + 5 6 7 ++ 177 196 170 ++ pcdpe/+ + 8 87 mu + 33 39 0 pcd + 9 + pe 49 37 3 + pe 9 mu pe 2 29 50 pcd pe 32 Total 261 301 223 Total 137 • m — marker. 17.2 ±1.8 33.7 ± 2 £ 12.7 ± 2 . 15,4 ±15 38.29 ± 5.21 31.84 ± 3.37 1.39 + 0.79 13.03 + 3.12 Figure 7. Map of Chr 13 summarizing all mapping data In this article. Sweet et al • MesenctiymaJ Dysplasia 9 3 of both proteins to regulatory stimuli such as diet and exercise. Reproductive failure in mes/mes males appears to be secondary to failure of the testes to descend normally, which may be due to occlusion of the inguinal canal by excess muscle. This conclusion is based on two observations. First, abnormalities in testis architecture and spermatogenesis, including cellular depletion, resemble those seen in cryptorchid testes of Dh/+ and Xpl/Y males (this article), XXY mice (Huckins et al. 1981), and other mammalian species rendered surgically cryptorchid (rhesus macaques, Resko et al. 1980; opossum, Scott et al. 1979; and rats, Jones et al. 1977) and in ipsilateral (right-sided) cryptorchidism in man (MIM #219050; GDB). Second, the degree of severity of the testicular abnormalities was related to the severity of cryptorchidism rather than to the genotype. Although the condition produced by the mes mutation does not precisely resemble a specific human syndrome, it has similarities with several. Of particular interest are genes that are located in the proximal region of the long arm of human Chr 5 (5qcen-q21) that is homologous to the region of mouse Chr 13 to which the mes gene maps (GDB 1994). Contractual arachnodactyly (CCA), which is a mutation in the fibrillin locus (FBN2) on human Chr 5q, has mesenchyme-derived tissue defects, including joint and skin laxity. This locus maps to human Chr 5q23-q31 at the distal end of the region homologous to mouse Chr 13; however, it has recently been mapped to Chr 2 in the mouse. Several spinal muscular atrophies also map to proximal 5q in the Chr 13 homologous segment. Four other human conditions that map to human Chr 5q have one or more of the features of mes/mes mutants: camptomelic dwarfism (CMD1), Treacher-Collins Syndrome, which is associated with deficient mesenchyme and facial dysmorphism, diastrophic dysplasia (DTD), and limb girdle muscular dystrophy (LGMD), which causes muscle weakness in the shoulder and hip girdles (GDB 1994). All four of these diseases, however, map to distal Chr 5q in the region homologous to mouse Chr 18. Loci that map in the mes region of Chr 13 and are potential candidates for the mes locus include Bmp6 (bone morphogenic protein; formerly Vgrl, Vg-1 related protein), Fgfr4 (fibroblast growth factor receptor 4), Rasa (ras p21 GTPase activating protein, also known as GAP), and Gasl (growth arrest specific 1). Bmp6 is a ho- 9 4 The Journal of Heredity 1996.87(2) mologue of Vg-1 in Drosophila and Xenopus and is a member of the transforming growth factor beta gene superfamily. It is involved in mesoderm initiation during embryogenesis (Lyons et al. 1989). Fgfr4 encodes one of the high affinity receptors for fibroblast growth factors, which appear to play an active role in mesoderm induction and in growth and differentiation of mesodermal and neuroectodermal cells (Givol and Yayon 1992). Although Bmp6 and Fgfr4 are the most promising candidates for mes, the present map position of mes is 10 to 15 cM distal to the locations of these two genes (Avraham et al. 1994; Dickinson et al. 1990). Rasa encodes a membrane protein that is associated with control of cell proliferation through regulation of normal ras p21 protein (Trahey and McCormick 1987). Gasl is one of several genes expressed at the G,, growth arrest phase of mammalian cells and is postulated to play a role in maintaining growth arrest (Schneider et al. 1988). A mutation in any of these genes might lead to uncontrolled growth in one or more tissues. More precise mapping of mes is in progress to enable us to test candidate loci. Mesenchymal dysplasia provides a new mouse model for studying connective tissue defects. Its pleiotropic effect on a variety of mesenchyme-derived tissues suggests mes may be a mutation in a gene involved in early mesenchyme formation or growth. Thus, determining the basic defect in mes/mes mutants should contribute to understanding early mesenchyme development. References Ashmore C, Parker W, Stokes H, and Doerr L, 1974. Comparative aspects of muscle fiber types In fetuses of the normal and "double-muscled" cattle. Growth 38: 501-506. Avraham KB, Givol D, Avlvl A, Yayon A, Copeland NG, and Jenkins NA, 1994. Mapping of murlne fibroblast growth factor receptors defines regions of homology between mouse and human chromosomes. Genomlcs 21:656-658. Baxter RC and Martin JL, 1989. Binding proteins for the Insulln-llke growth factors: Structure, regulation and function. Prog Growth Factor Res 1:49-68. Cattanach BM, et al. 1994. A new reciprocal translocatlon with pleiotropic effects. Mouse Genome 92:513. Davtsson MT, 1990. The Jackson Laboratory Mouse Mutant Resource. Lab Anlm 19:23-29. Deol MS and Green MC, 1966. Snell's waltzer, a new mutation affecting behaviour and the Inner ear of the mouse. Genet Res 8:339-345. Dickinson ME. Kobrin MS, Sllan CM, Klngsley DM, Justice MJ, Miller DA, Cecl JD, Lock LF, Lee A, Buchberg AM, Siracusa LD, Lyons KM, Derynck R, Hogan BLM, Copeland NG, and Jenkins NA, 1990. Chromosomal localization of seven members of the murine TGF-beta superfamily suggests close linkage to several morphogenetic mutant loci. Genomlcs 6:505-520. Donahue LR and Beamer W, 1993 Growth hormone deficiency In 'little' mice results In aberrant body composition, reduced Insulin-like growth factor-! (IGF1) and insulln-Uke growth factor-binding proteln-3 (IGFBP3), but does not affect IGFBP-2, -1, or -4. J Endocrlnol 13631-104. Donahue LR, Hunter SJ, SherbJom AP, and Rosen C, 1990. Age-related changes In serum Insulln-llke growth factor-binding proteins In women. J Clln Endocrlnol Metab 71:575-579. GDB, 1994. GDB, Human Genome Database at Johns Hopkins University, Baltimore, Maryland. October 1, 1994, at 10:00 am Eastern Time. Givol D and Yayon A, 1992. Complexity of FGF receptors: genetic basis for stuctural diversity and functional specificity. FASEB J 63362-3369. Green EL, 1985. Tables and a computer program for analyzing linkage data. Mouse News Lett 73:20-22. Green MC, 1952. A rapid method for clearing and stainIng specimens for the demonstration of bone. Ohio J Scl 52:31-33. Green MC, 1989. Catalog of mutant genes and polymorphic loci. In: Genetic variants and strains of the laboratory mouse, 2nd ed (Lyon MF and Searle AG, eds). Oxford: Oxford University Press; 12-404. Green MC, Sweet HO, and Bunker LE, 1976. Tlght-skln, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol 82:493507. GrOneberg H, 1952 The genetics of the mouse, 2nd ed. The Hague: Niljoff. Hollander WF, 1981. Linkage relations of dumpy, a recessive mutant on chromosome 13 of the mouse. J Hered 72:358-359. Huckins C, Bullock LP, and Long JL, 1981. Morphological profiles of cryptorchid XXY mouse testes. Anat Rec 199:507-518. Jimenez SA, Williams CJ, Myers JC, and Bashey Rl, 1986. Increased collagen biosynthesis and Increased expression of type I and type III procollagen genes In tight-skin (TSK) mouse fibroblasts. J Blol Chem 261657-662. Johnson DR, 1986. The genetics of the skeleton. Animal models of skeletal development. Oxford: Oxford University Press. Jones TM, Anderson W, Fang VS, Landau RL, and Rosenfield RL, 1977. Experimental cryptorchidism In adult male rats: hlstological and hormonal sequelae. Anat Rec 189:1-28. Laemmll UK, 1970. Cleavage of structural proteins during the assembly of the head of the bacterlophage T4. Nature 227.680-682. Les EP, 1991. Reproductive performance, 1988, Table 2.5. In: Handbook on genetically standardized JAX mice, 4th ed (Green MC and Wltham BA, eds) Bar Harbor, Malne:The Jackson Laboratory; 37. Little TM and Hills FJ, 1975. Transformations. In: Statistical methods In agricultural research (Little TM and Hills FJ, eds). Davis: University of California Press; 103120. Lyon MF and Meredith R, 1969. Muted, a new mutant affecting coat colour and otollths of the mouse, and Its position in linkage group XfV. Genet Res 14:163-166 Lyons K, Graycar JL, Lee A, Hashml S, Undqulst PB, Chen EY, Hogan BLM, and Derynck R, 1989. Vgr-1, a mammalian gene related to Xenopui Vg-1, Is a member of the transforming growth factor 8 gene superfamily. Proc Nat! Acad Sci USA 86:4554-4558. Mather K, 1946. Statistical analyses in biology, 2nd ed. New Yort Intersclence Publishers. MGD (Mouse Genome Database), 1994. Mouse genome Informatics project. The Jackson Laboratory, Bar Harbor, Maine. World Wide Web (URUhttp://www. lnformatics.jax.org/). Pell JM and Bates PC, 1992. Differential actions of growth hormone and lnsulln-llke growth factor-! on tls^ ,P<S>9elinQSetabOllSm '" d W 3 r f ""Ce" E n d o c r 1 n o l o g y lJU.m,!-l»5U. Resko JA. Jackson GL, HucHns C, Stadelman H, and Spies Ha 1980. Cryptorchld rhesus macaques: long term studies on changes In gonadotroplns and gonadal steroids. Endocrinology ,07:,.27-n3, Schneider C, King RM, and Phlllpson U 1988. Genes specifically expressed at growth arrest of mammalian cells. Cell 54:787-793. "luxold" mutants In the house mouse. Genet Res 5:171197. retlc transfer of proteins from polyacrylamlde gels to nitrocellulose sheets: procedure and some appllca- Sweet HO and Bronson RT, 1988. Mouse News Lett 81: Sweet HO and Green MC, 1981. Progressive ankytosis, a new skeletal mutation In the mouse. J Hered 72:8793 HQ ^ « P 0 . a new mutation In the mouse. J Hered 71:207""• Tomas FM.KnowlesSE; Owens PC Read LC, Chandler t l o n s PTOC N a t l A c a d ^ USA 7 6 : 4 3 5 ( M 3 5 6 Trahey M and McCormlck F, 1987. A cytoplasmlc pro' ? " simulates normal fern p21 GTTPase but does not a f f e c t onco nlc ^ mutants. Science 238542-545. Wakasugl S, Iwanaga T, lnomoto T, Tengan T, Maeda S, V ^ ^ ^ A S S ^ £ K ^ o ( ( a c ) a | d e v e l o p m e n t l n a t r a n s g e n | c m o u s e . Dev Genet 9203-212 ^ ^ m 19gg M a ] [ o r m a U o n s y n d r o m e s : a ^ e w 0, ggSfi^^g^i^^ ^ m u J c l e p r o t e | n me t a bollsm In nltrogen-restrlcted rats. J Endocrlnol 128^7-105. mouse^uman homo.ogy. J Med Gene, 25:480-487. Received February 22, 1995 Accepted September 6, 1995 70 Scot, JN. Fritz HI, and Nagy F, ,979. Response to cryptorchidism of the testls and epkildymls of the opossum (Didelphis virgwiond). J Reprod Fert 57:175-178. t r o g e n balance Searie, AG, 1964. The genetics and morphology of two Towbln H, Staehelln T, and Gordon J, 1979. Electropho- Corresponding Editor Christine Kozak Sweet et al • Mesenchymal Dysplasia 9 5