* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download one-step and stepwise magnification of a bobbed lethal
Cell-free fetal DNA wikipedia , lookup
Non-coding RNA wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Genealogical DNA test wikipedia , lookup
DNA supercoil wikipedia , lookup
Human genome wikipedia , lookup
History of genetic engineering wikipedia , lookup
Comparative genomic hybridization wikipedia , lookup
Medical genetics wikipedia , lookup
Segmental Duplication on the Human Y Chromosome wikipedia , lookup
Genome evolution wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Genomic library wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genomic imprinting wikipedia , lookup
Hybrid (biology) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Designer baby wikipedia , lookup
Gene expression programming wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Microevolution wikipedia , lookup
Genome (book) wikipedia , lookup
Y chromosome wikipedia , lookup
X-inactivation wikipedia , lookup
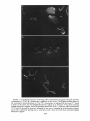
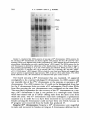
Copyright 0 1986 by the Genetics Society of America ONE-STEP AND STEPWISE MAGNIFICATION OF A BOBBED LETHAL CHROMOSOME IN DROSOPHILA MELANOGASTER SHARYN A. ENDOW AND DONALD J. KOMMA Department of Microbiology and Immunology, Duke University Medical Center, Durham, North Carolina 27710 Manuscript received April 28, 1986 Revised copy accepted July 14, 1986 ABSTRACT Bobbed lethal (bb‘) chromosomes carry too few ribosomal genes for homozygous flies to be viable. Reversion of bb‘ chromosomes to bb or nearly bb+ occurs under magnifying conditions at a low frequency in a single generation. These reversions occur too rapidly to be accounted for by single unequal sister chromatid exchanges and seem unlikely to be due to multiple sister strand exchanges within a given cell lineage. Analysis of several one-step revertants indicates that they are X-Y recombinant chromosomes which probably arise from X-Y recombination at bb. The addition o f ribosomal genes from the Y chromosome to the bb’ chromosome explains the more rapid reversion of the bb’ chromosome than is permitted by single events of unequal sister chromatid exchange. Analysis of stepwise bb’ magnified chromosomes, which were selected over a period of 4-9 magnifying generations, shows ribosomal gene patterns that are closely similar to each other. Similarity in rDNA pattern among stepwise magnified products of the same parental chromosome is consistent with reversion by a mechanism of unequal sister strand exchange. C + HROMOSOMES that carry severe deficiencies of the 18s 28s ribosomal RNA genes are referred to as bobbed lethal (bb’) chromosomes (RITOSSA, ATWOODand SPIEGELMAN 1966). bb’ chromosomes contain too few ribosomal genes for bbl homozygotes to be viable. T h e number of ribosomal genes on a bb’ chromosome must therefore increase more than twofold in order for the chromosome to serve as the sole source of rDNA in a fly. Progeny of flies which carry a low number of ribosomal genes show an improvement in phenotype relative to their bobbed (bb) parents. This reversion of the bb phenotype has been termed “magnification” and is correlated with an increase in rDNA in the bobbed mugnijied (bb”) chromosomes (RITOSSA 1968). Evidence from studies on ring chromosomes favors unequal sister strand exchange as the major mechanism of ribosomal gene increase during magnification (TARTOF 1973b, 1974; ENDOW,KOMMA and ATWOOD1984; D. J. KOMMAand K. C. ATWOOD,unpublished results). This evidence includes the observations that magnification is inhibited in bb alleles in ring chromosomes (TARTOF1973b, 1974; D. J. KOMMAand K. C. ATWOOD,unpublished results) Genetics 1 1 4 511-523 October, 1986. 512 S. A. ENDOW AND D. J. KOMMA and that ring chromosomes carrying bb alleles are lost under magnifying conditions compared with nonmagnifying conditions (ENDOW,KOMMA and ATWOOD 1984). Loss of ring chromosomes under magnifying conditions is correlated with an increase in abnormal ring structures, mostly dicentric chromosomes, that are attributed to products of sister chromatid exchange (ENDOW, KOMMAand ATWOOD1984). Evidence consistent with an extracopy hypothesis of rDNA magnification is the finding of extrachromosomal rDNA rings in testes of magnifying males (GRAZIANI, CAIZZIand GARGANO 1977), instability and RITOSSA1970; BONof newly magnified bb (RITOSSA1968; HENDERSON CINELLI et al. 1972; LOCKER1976) and the finding of amplified rDNA in GO magnifying males (RITOSSAet al. 1971; LOCKERand MARRAKECHI1977; DE CICCOand GLOVER1983). These observations are not unequivocal in their support of an extracopy mechanism of magnification, however, since they can also be explained within the context of an exchange hypothesis: The rDNA rings could arise as products of intrachromatid excision (ENDOW,KOMMAand ATWOOD1984), the instability could be due to chromosomes with which the magnified allele is combined rather than to the magnifying event, and the amplified rDNA might not contribute to the magnified phenotype. In contrast, evidence from ring chromosome studies is not easily explained by the alternate hypothesis: The failure of ring Xbb chromosomes to magnify cannot be easily accounted for by the extracopy hypothesis since extracopies of rDNA should be produced and integrated equally well into rod or ring X b b chromosomes, whereas a lower recovery of bb" is predicted for ring Xbb chromosomes if magnification occurs by an unequal sister strand exchange mechanism. This is because sister chromatid exchange in ring chromosomes results in the formation of dicentric chromosomes and interlocked rings which are lost from the cells that bear them (MCCLINTOCK 1938, 1941). The finding of the predicted cytological structures and the observation that ring X b b chromosomes are lost under magnifying conditions (ENDOW,KOMMA and ATWOOD1984) therefore support unequal sister chromatid exchange as the major mechanism of magnification. Some events that result in bb", however, are not easily explained by a sister strand exchange mechanism of magnification: Under magnifying conditions bb' chromosomes revert in a single generation with low but measureable frequencies to bb or nearly bb+ (ATWOOD1969; RITOSSA1972). These onestep bobbed lethal magnified (bb'") chromosomes can be selected by their ability to produce viable flies together with an X or Y chromosome that is completely or almost completely rDNA-deficient. Magnification of bb' chromosomes to bb or nearly bb+ in a single generation cannot be due to single unequal sister chromatid exchanges, since they can, at most, almost double the starting number of ribosomal genes when pairing is in the most extreme register. The bb' chromosomes must more than double their ribosomal genes in order to support viability as the sole source of rDNA for the fly. One-step reversion of bb' chromosomes might occur by one or more of several mechanisms including X - Y recombination, as suggested by ATWOOD(1969), or by some type of productive rDNA replication. A third possibility is that multiple 513 BOBBED LETHAL MAGNIFICATION unequal sister strand exchanges occur in the same stem cell or premeiotic cell lineage, producing bb' reversions in a single generation. We consider this unlikely to account for the one-step bb' reversions, however, since they are usually recovered as single or low frequency (and therefore presumably meiotic) events in individual bottles (ATWOOD1969; RITOSSA1973). We have recovered and analyzed several one-step bb'" chromosomes. Our results indicate that one-step bb'" chromosomes are X - Y recombinant chromosomes which probably arise as a result of X-Y recombination at bb. These recombinational events can occur premeiotically or meiotically and are increased in frequency under magnifying conditions compared with nonmagnifying conditions. They represent a second, although low frequency, mechanism to account for ribosomal gene increase during magnification and provide an explanation for reversion of bb' chromosomes more rapidly than is permitted by single events of unequal sister strand exchange. During the course of this study we also analyzed several stepwise bb'" chromosomes. These stepwise bb' reversions were magnified in separate lineages for 4-9 generations. Southern blot analysis of the final complement of ribosomal genes present in six stepwise bb'" chromosomes from two different lineages shows ribosomal gene patterns which are very similar to one another and independent of lineage. This similarity in rDNA pattern among stepwise magnified products of the same parental chromosome is consistent with an unequal sister chromatid exchange mechanism of reversion. MATERIALS AND METHODS Drosophila stocks: Stocks of D.melanogaster carrying the y bb' and Ybby+chromosomes were cloned in June, 1984 and October, 1982, respectively. T h e y bb' chromosome was tested for lethality in homozygous females; n o y bb females were recovered. One-step bb'" chromosomes were magnified in males and recovered in either females or males according t o one of the following schemes. For recovery in females, y bb'/Ybb magnif y F g males were mated to + / Z n ( l ) s ~ ~ ~ys sc4 c ~ sc8 ~ , car females. T h e Zn(1)sc4 2scBR,y sc4 sc car chromosome is an X-No chromosome; it is referred to as sc4sc8. Any y females recovered among the progeny were expected t o be sc4scB/y bb'". These females were tested for segregation of the bb and car markers by mating to sc4sc8/y+Y males, and the y bb'" chromosomes were maintained as stocks of C(l)DX, y/y bb'"/y+Y. For recovery of one-step bb"" chromosomes in males, y bb'/Ybby+ males were mated to C(l)RM, y 'U bb/ Ybb- females. T h e Ybb- chromosome carries a low number of ribosomal genes (TARTOF 1973a; SPEAR 1974; ENDOW 1982b) and has an g t i m o r p h i c effect with respect to bb (MULLER1932). T h e C(1)RM chromosome is an XX chromosome. Any y male progeny were expected to be y bbfm/Ybb-and were stocked as described above and tested further. An experiment to test for one-step reversion of bb' chromosomes under nonmagnifying conditions was carried out by mating y bb'/Yy+ nonmagnifying males t o +/sc4sc8 tester females. In order to recover stepwise bb" chromosomes, individual bb'/Ybby+ males were mated each generation in single pairs to C(l)RM, y2 wa su(w")/Y2y + females in order t o continue the magnification and also to sc4sc8/sc4sc8/BsYy+ tester females. y bb'/Y'bj+ males which showed an improvement in phenotype with respect to their fathers were selected whenever possible in each successive generation to continue the magnification. y bb'" chromosomes which were recovered in females with the sc4sc8 chromosome were stocked and tested further. T h e lineage with respect to vial number of each of the stepwise 66'" chromosomes that was recovered was recorded. + 514 S. A. ENDOW AND D. J. KOMMA T h e one-step bbIm chromosomes were tested for the presence of the Y chromosome on the basis of fertility as X/O, X/y+YLand X / O ; T(Ys;4)/4males. T h e y+YL chromosome (MULLER1948; NOVITSKI1952; SANDLER1954) was obtained from Bowling Green. Males carrying y'YL but not Ys are sterile. T h e T(Ys;4) chromosome is a spontaneous translocation recovered by D.J.K. It is Ks+,bb and homozygous lethal. Males carrying T(Ys;4) but not YL are sterile. Southern blot hybridizations: Crosses to produce sc4sc8/y bb" females were made by mating y bb'"/y+Y males to +/sc4sc8 females in single pairs. Arpropriate crosses were also made to produce y bb'/Ybby+Go magnifying males, sc4sc8/Y ?+ males, C(l)DX/Ybby+ females, y bb'/Y males, C( l)DX/Y females and +/y bb' females. Neuroblasts and imaginal discs were dissected from y third instar female larvae, and DNA was prepared, as described previously (ENDOWand GLOVER1979; ENDOW1982a), from pooled samples of 3-15 sets of brains and discs and from 3-5 individuals from each vial. In one case, DNA was prepared from adult brains in order to avoid sampling nondisjunctional offspring. Pooled and individual samples of DNA were prepared from 2-3 single pair matings for each genotype that was examined. rDNA blot patterns for pooled samples were compared with each other and with patterns for individual samples for each of the one-step and stepwise bb" chromosomes that were examined. T h e individual samples were used to confirm the rDNA blot patterns for each chromosome and to exclude the possibility of tissue from contaminating genotype in the pooled samples. Ribosomal gene repeats attributed to the y bb' chromosome were inferred from comparisons of the rDNA blot patterns for bb'lyo' and C(I)DX, yf/YO', X°K/y bb' and X°K/XoK,and y bb'/Y*?+ and C(I)DX, y wQ/Ylby+o r sc4sc8/Ybby+flies. T h e yo' and X°K chromosomes are from o u r stocks of D. melanogaster Ore-R and OK- 1 (ENDOWand GLOVER1979; ENDOW 1980), respectively. T h e C(I)DX chromosome is an attached X chromosome that is deficient for the nucleolus organizer re ion. T h e Ybby+rDNA pattern was obtained by examining DNA from tissue of sc4sc8/Y y males o r C(l)DX, y wa/Ybby+females. E+ DNA was digested with EcoRI o r BamHI restriction endonuclease, fractionated on 0.8% agarose gels, transferred to nitrocellulose and hybridized with ['*P]rDNA as described previously (ENDOW1980; ENDOW1982a). T h e rDNA probe was the 11.5-kb intron- insert from pDmraa51 1 (DAWID,WELLAUER and Long 1978; ENDOW1982a). DNA quantitation: T h e concentrations of purified DNA in samples from pooled tissue used for the Southern blot in Figure 2 were determined by ethidium bromide fluorescence as described (MANIATIS,FRITSCHand SAMBROOK 1982), using bacteriophage lambda DNA samples of known concentration as standards. Briefly, 2-10 ng of each DNA in a volume of 5 111 was mixed with 5 p1 of 2 pg/ml ethidium bromide in distilled water; samples were photographed under shortwave ultraviolet illumination onto Kodak Tri-X sheet film. Photographs were scanned using a Zeineh soft laser scanning densitometer. Average maximum peak heights were determined for two scans of each sample. A linear response of film density to ethidium bromide fluorescence was found for DNA concentrations of 0.1-1.0 pg/ml. T h e amount of DNA in each sample was determined relative to the bacteriophage lambda standards; the concentration of DNA in each sample was calculated from the amount of DNA and the volume sampled. Cytological preparations: Tissue squashes were prepared from third instar larval neuroblasts from one-step and stepwise y bb'"/y+Y males, y bb'/BSY males, C(I)RM, y2 w0 su(w")/Y"y' females and B/Ybby+males by dispersing the tissue briefly in a drop of 45% acetic acid on a siliconized coverslip and then squashing firmly onto a Denhardt-treated slide (BRAHICand HAASE1978). Coverslips were flipped off after freezing in liquid nitrogen, and slides were fixed overnight in absolute ethanol. Preparations were stained Boehringer-Mannheim) and scanned under with DAPI (4',6-diamidino-2-phenylindole; fluorescence as described previously (ENDOW,KOMMAand ATWOOD1984). RESULTS Frequency of one-step reversion under nonmagnifying conditions: An experiment was carried o u t to determine the frequency of spontaneous one-step 515 BOBBED LETHAL MAGNIFICATION TABLE 1 Frequency of one-step bb' reversion under nonmagnifying conditions Offspring I. Recovery in females +? Y? dd % bb" 14,756 0 20,236 <0.0068% Progeny from cross of y bb'/Yy+ nonmagnifying males to +/In( I ) S C % ~ ~ , y sc4 sc8 cur IUI) females. The one-step bb'" are recovered as y females; no y females were recovered in this experiment. TABLE 2 Recovery of one-step bb'"' chromosomes under magnifying conditions Offspring I. Recovery in males 11. Recovery in females 2 3666 +? Y? +d 7849 22 7716 Progeny from crosses of y bb'/Y"y+ magnifying males to C(I)RM, y U bb/Ybb females (I) or +/Zn(I)sc'"~c~~, y sc4 sc8 cur females (11). The onestep y bb" are recovered as y males (I) or y females (11). Twenty of the 22 y females in I1 arose in a single bottle. reversion of bb' chromosomes. Nonmagnifying y bb'/Yy+ males were mated to +/sc4sc8 tester females carrying the rDNA-deficient sc4sc8 chromosome. Offspring were examined for the occurrence of y females which were expected to be y bb'"/sc4sc8. N o y females were recovered among 14,756 females that were examined (Table l), indicating that the frequency of bbl reversion under nonmagnifying conditions is <0.0068%. One-step bb" chromosomes: One-step y bb" chromosomes were magnified for one generation in males and recovered in either males or females (Table 2). Selection of one-step y bb" chromosomes was by viability with an rDNAdeficient X or Y chromosome. Five independent one-step y bb'" chromosomes were obtained from 11,539 flies that were screened. Two of the one-step y bb'" chromosomes were recovered in males. One of these was sterile and could not be examined further; the second was designated 10. Three one-step y bb" chromosomes (35, 45 and 48) were recovered in females. Of these three, one (48) was part of a cluster of 20 individuals from the same bottle. Three members of the cluster were saved and analyzed as described below. Results of this analysis are consistent with the conclusion that the cluster of flies carrying onestep y bb" chromosomes arose from the same premeiotic event. T h e recovery of 20 individuals in a single cluster indicates that the premeiotic event occurred before the first mitotic division in sperm cell differentiation, i.e., during stem cell division or differentiation. A total of four independent one-step y bb'" chromosomes was analyzed. 516 S. A. ENDOW AND D. J. KOMMA Cytological and molecular analysis of one-step bb Inr chromosomes: Squashes of larval neuroblasts were prepared, stained with the fluorochrome DAPI and scanned under fluorescence for metaphase figures. The y bb' chromosome is a typical rod-shaped X chromosome (Figure la) in which the short arm is sometimes visible. The Ybby+chromosome consists of a long arm with three brightly fluorescent regions and a short arm with two brightly fluorescent regions (Figure lb). Similar observations have been made for other Y chromosomes stained with Hoechst 33258 or quinacrine (GATTI, PIMPINELLI and SANTINI1976; GATTI and PIMPINELLI1983). Three of the one-step y bb" chromosomes, 10, 35 and 48, appeared as acrocentric chromosomes with a uniformly staining long arm and a short arm consisting of three brightly fluorescent regions (Figure IC). T h e three chromosomes from the cluster of 20 y bb'" flies, 48-1, 48-3 and 48-4, were examined independently. Since they showed the same cytological structure and genetic behavior, they are referred to as 48. These one-step bb'" chromosomes, 10, ?5 and 48, were interpreted to be X-Y recombinant chromosomes from their cytological appearance. Genetic tests confirmed that all three one-step y bb'" chromosomes carried YL since they produced fertile males in combination with T(Ys;4), but not with y'YL or in the absence of a Y chromosome. These cytological and genetic data indicate that 10, 35 and 48 arose by an X-Y recombinational event that transferred Y L to the X chromosome. The Southern blot hybridization rDNA patterns for the one-step y bb'" chromosomes were compared with the Ybby+,y bb' and y bb'/Ybby+ rDNA patterns (Figure 2). This analysis indicated that part or all of the bb locus from the Ybby+chromosome was present in the X-Y recombinant chromosomes. The rDNA patterns for one-step y bb'" 10, 35 and 48 differed from one another; however, the rDNA patterns for 48-1, 48-3 and 48-4 were identical to one another. This observation, together with the cytology and genetic behavior of 48-1, 48-3 and 48-4, indicates that they most likely arose from the same reczmbinational event. Figure 2 (lanes a and b) shows the rDNA patterns for XX-,/Ybby' and y bb'/Ybby+flies. The rDNA bands in lane b attributed to the y bbl chromosome are indicated with open circles. T h e y bb' chromosome was also examined with the X°K and YOr chromosomes in order to confirm the assignment of rDNA bands to the y bb' chromosome. It was necessary to derive the y bb' rDNA pattern by subtraction of the rDNA bands from the bb or bb+ homologue, because the y bb' chromosome is lethal in homozygous form and also with X-NO chromosomes, which would otherwise have been used for this analysis. rDNA patterns for the one-step y bb'" chromosomes shown in Figure 2 are with an X-NO chromosome. The three one-step y bb'" chromosomes which appeared cytologically to be X-Y recombinant chromosomes (10, 35 and 48) contain rDNA bands characteristic of both the y bb' and the Ybby+chromosomes (Figure 2, lanes c-e). One-step bb'" 48 (lane e) contains only a few ribosomal genes that can be attributed to the y bb' chromosome, while I 0 and 35 contain more. Repeats derived from the Ybby+chromosome are readily apparent in all three one-step bb'" chromosomes. The DNAs in lanes b-e are present in approximately equal amounts. FIGURE1.-Cytological structure of one-step y bb'" chromosomes compared with y bb' and p y + chromosomes. a, The y bb' chromosome is indicated by the arrow. The brightly staining region is the centromeric heterochromatin. b, The Y"y' chromosome is indicated by the arrow. Ys,which is closer to the arrow, has two brightly fluorescent regions at its telomere; YL has three terminal brightly fluorescent regions. The X chromosome shown here is bb+. c, One-step y bb'" chromosomes 10, 35 and 48 showed the structure indicated by the arrow, consisting of an acrocentric chromosome with a uniformly staining arm, a centromere and a short arm with three terminal brightly fluorescent regions. 517 518 S. A. ENDOW AND D. J. KOMMA 7 kb 1.5 kb h-4 FIGURES.--Southern blot rDNA patterns of one-step y bb" chromosomes. rDNA patterns for the one-step y bb" chromosomes are shown together with patterns for the y bb' and u"y+ chromosomes. DNAs were digested with EcoRI. fractionated on a 0.8% agarose gel and transferred to nitrocellulose. Hybridization was with a purified intron- rDNA repeat. The rDNA pattern for the y bb' chromosome is derived by subtraction of the bands present in lane a from those in l z e b. The positions corresponding to 11.5- and 17-kb repeats are indicated. DNAs are from a, XXw/ P y + ; b, y b b ' p y * ; c, one-step y bb" IO; d, one-step y bb" 3%e, one-step y bb" 48; and f. pDmr. a5 1 1 (1 1.5-kb intron- rDNA repeat). DNAs in lanes b-e are from larval brains and imaginal discs and are present in approximately equal amounts; DNA in lane a is from adult brains. The rDNA bands attributed to the y bb' chromosome are indicated with open circles in lane b. T h e fourth one-step y bb" chromosome that was examined, 45, appeared cytologically to be a normal rod-shaped X chromosome. Its rDNA pattern did not resemble that of the Ybby+ chromosome or the stepwise or one-step y bb" chromosomes. T h e rDNA pattern for 45 could not be distinguished from that for the X chromosome from our strain of D. melunogustet Ore-R when DNAs from flies carrying the two chromosomes were compared on the same filter. T h e most likely explanation for the recovery of the Xor chromosome as a onestep y bb" chromosome is that it was present in a nonvirgin tester female which had mated with an Xo'/BsYy+ sibling male from the same cross from which she arose. T h e Xor chromosome must have undergone a spontaneous mutation to y in order for it to have been recovered in our screen, and the female carrying the chromosome could not have been very fertile, because no BS progeny were recovered from the bottle that produced 45. Stepwise bb" chromosomes: Stepwise y bb" chromosomes were magnified in males and recovered in females carrying an X chromosome deficient for bb. BOBBED L E T N A L MAGNIFICATION 519 FIGURE3.-Southern blot rDNA patterns of stepwise y bbh chromosomes. rDNA patterns for stepwise y bb" flies from two different lineages are shown. DNAs are grouped to show similarities in rDNA patterns for flies from different lineages. DNAs were digested with EcoR1, fractionated on a 0.8% agarose gel and hybridized with a purified intron- rDNA repeat as described in MATERIALS AND METHODS. Lane f was from another gel. The lines indicate the bands which correspond to those in lane f. DNAs are from stepwise y bb": a, 1-2-3-3-4; b. 6-2-3-1; c, 6-2-3-13-3-2-2-2; d, 6-2-3-1-2-1. e, 1-2-4-1-2 and f, 6-2-3-2. Six lines of stepwise y bb" chromosome were established from single flies arising from successive single-pair matings. These lines were designated 6-2-31, 6-2-3-2, 1-2-4-1-2, 1-2-3-3-4, 6-2-3-1-2-1 and 6-2-3-1-3-3-2-2-2, with successive numbers indicating the vial for each generation and the total numbers indicating the number of magnifying generations. T h e six lines represent two lineages of y bb" chromosomes which were carried in different individuals and 1-2-3-34), three (6-2-3-1 and 6-2-3-2) or four after the first two (1-2-4-1-2 (6-2-3-1, 6-2-3-1-2-1 and 6-2-3-1-3-3-2-2-2) generations. Stepwise y bb" chrowas recovered in an X-No/y bb" female after four magnifying mosome 6-2-3-1 and 6-2-3-1-3, were generations; her half-sib y bb'/Ybby+ brothers, 6-2-3-1-2 fathered by the same male but apparently carried y bb' chromosomes of lower rDNA redundancy than she did since they required two and five additional generations, respectively, to produce x-NO/y bb" females. Cytological and molecular analysis of stepwise bb" chromosomes: T h e six stepwise y bb" chromosomes were examined cytologically, and all six were found to be normal rod-shaped X chromosomes. T h e rDNA patterns for the six stepwise y 66" chromosomes are shown in Figure 3. T h e rDNA patterns for the six chromosomes are very similar, but not identical, to one another. T h e small differences detectable among the rDNA patterns are due primarily to different intensities of repeats in the 10to 12.5-kb range. There are also some rDNA bands of 4 0 k b that are present in some patterns (a-c) but not others (d-9. No lineage-specific differences were detected among the six stepwise bb" chromosomes examined in this study. 520 S. A. ENDOW AND bb' bby + D. J. KOMMA bb " iy+ \ + Y Y FIGURE4.-Origin of one-step bb'" chromosomes by X-Y recombination. One-step magnification of bb' chromosomes can be accounted for by recombination between the X and Y chromosomes at bb to produce X-Y recombinant chromosomes carrying YL. Similarity in rDNA pattern between a starting bb chromosome and its magnified products has been reported previously (DE CICCOand GLOVER1983); in this previous study, however, no differences in rDNA pattern among individual flies were found after six magnifying generations. DISCUSSION O u r genetic, cytological and molecular analysis of three one-step bb" chromosomes indicates that they arose by an X-Y recombinational event in which YL and bb, but not KS,were transferred to the X chromosome with the loss of y+. Figure 4 shows a schematic diagram of an X-Y recombination that could account for the chromosomes that we recovered. Because we detect different amounts of X chromosome ribosomal genes in the rDNA patterns for the recombinant chromosomes, we believe that the recombinations occurred at bb. Our molecular analysis indicates that the recombinant rDNA patterns contain repeats from both the starting X and Y chromosomes. The addition of ribosomal genes from the Y chromosome to the bbl chromosome explains the more rapid reversion of the bb' chromosome than is expected for a single step of unequal sister chromatid exchange. Our observation that reversion of a bbl chromosome involves X-Y recombination indicates that bb reversion during magnification may occur by a mechanism of homologous recombination in addition to the previously described sister chromatid exchange (TARTOF 1973b, 1974; ENDOW,KOMMA and ATWOOD1984). The experiments reported here demonstrate that both one-step and stepwise bb" chromosomes can be recovered from flies of the same magnifying genotype. One-step bbim arises by X-Y recombination, probably at bb, whereas step- BOBBED LETHAL MAGNIFICATION 52 1 wise bb'" most likely arises as a result of unequal sister chromatid exchange in successive generations. The Ybb-chromosome, which is frequently used to establish magnifying conditions, is not required for magnification; other Ybb chromosomes, such as the Ybby+chromosome used in these experiments, also result in magnification. The observation that other Ybb chromosomes will result in magnification has been reported previously (ATWOOD1969; BONCINELLI et al. 1972; HAWLEY and TARTOF 1985; D. J. KOMMAand S. A. ENDOW,unpublished results; D. J. KOMMAand K. C. ATWOOD,unpublished results). We observed a frequency of 0.035% for one-step bb' reversion (Table 2), which is lower than previously reported values of 0.13-0.25% (ATWOOD1969; RITOSSA1972, 1973). This might be due to our use of a more severe X and Y combination than the ones used by others or to our ability to account for clustering. We obtained 20 bb'" from a single bottle; cytological and molecular analysis of three of the 20 suggests that the 20 bb" arose from a single premeiotic event. Clear clustering was not obtained in previous experiments; however, if some of the one-step bb'" flies obtained previously represented clusters or portions of clusters, the frequencies of one-step events reported previously would be overestimates. A control experiment that we carried out resulted in a frequency of spontaneous bbl reversion of <0.0068%. The frequency of bb' reversion under magnifying conditions is therefore increased at least fivefold compared with nonmagnifying conditions. Thus, the recombinational events that result in one-step bb' reversion are increased under magnifying conditions compared with nonmagnifying conditions. Our data cannot be compared with frequencies reported by others for X-Y recombination under magnifying or nonmagnifying conditions, because only a subset of the total recombinants were recovered, i.e., those that carried sufficient rDNA for viability with an rDNAdeficient X or Y chromosome. Recovery of 20 bb" that probably correspond to a single event indicates that the recombinational events resulting in one-step bb'" can occur early in spermiogenesis, i.e., in predefinitive stem cell divisions or during stem cell differentiation. Three other one-step bb'" chromosomes that were recovered probably arose during meiosis because they arose as single events in individual bottles. The fifth chromosome recovered as a one-step bbim was a contaminating chromosome that was probably transmitted by a nonvirgin female. This chromosome must have undergone a spontaneous mutation to yellow in order to have been recovered in our screen. T h e six stepwise bb" chromosomes that we examined showed very similar but nonidentical rDNA patterns. Similarity in rDNA pattern among magnified products is consistent with the generation of stepwise bb'" by sequential sister chromatid exchange in successive generations. The small differences in rDNA pattern that we observe for different stepwise bb" chromosomes can be attributed to different sites of exchange for events in each lineage. In addition, new repeats could arise from unequal sister strand exchange at repetitive sites that lie within a repeat, e.g., the internal repeats present in the nontranscribed spacer regions of the Drosophila ribosomal genes (LONGand DAWID1979). In these experiments we selected for sequential magnification products carrying 522 S. A. ENDOW AND D. J. KOMMA an increased number of repeats. T h e prediction of crossover models in which both increases and reductions in repeat number can occur is that the tandem array will become homogeneous with respect to repeat type (SMITH 1973; SZOSTAK and Wu 1980). We are currently using molecular methods to examine the effect of alternating cycles of magnification and reduction on a ribosomal gene array. These studies were supported by a grant from the National Institutes of Health (GM 31279) to S.A.E. S.A.E. is a recipient of a United States Public Health Service Research Career Development Award (HD 00433). We thank K. C. ATWOODfor helpful discussions during the early part of these studies. LITERATURE CITED ATWOOD,K. C., 1969 Some aspects of the bobbed problem in Drosophila. Genetics 61 (Suppl.): 319-327. BONCINELLI, E., F. GRAZIANI, L. POLITO,C. MALVAand F. RITOSSA,1972 rDNA magnification at the bobbed locus of the Y chromosome in Drosophila m.elanogaster. Cell Differ. 1: 133-142. BRAHIC,M. and A. T. HAASE,1978 Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc. Natl. Acad. Sci. USA 75: 6125-6129. DAWID,I. B., P. K. WELLAUER and E. 0. LONG,1978 Ribosomal DNA in Drosophila melanogaster I. Isolation and characterization of cloned fragments. J. Mol. Biol. 126 749-768. DE CICCO,D. V. and D. M. GLOVER,1983 Amplification of rDNA and type 1 sequences in Drosophila males deficient in rDNA. Cell 32: 1217-1225. ENDOW,S. A., 1980 On ribosomal gene compensation in Drosophila. Cell 22: 149-155. ENDOW,S. A., 1982a Polytenization of the ribosomal genes on the X and Y chromosomes of Drosophila melanogaster. Genetics 100 375-385. ENDOW,S. A., 1982b Molecular characterization of ribosomal genes on the Ybb- chromosome of Drosophila melanogaster. Genetics 102: 9 1-99. ENDOW,S. A. and D. M. GLOVER,1979 Differential replication of ribosomal gene repeats in polytene nuclei of Drosophila. Cell 17: 597-605. ENDOW,S. A., D. J. KOMMAand K. C. ATWOOD,1984 Ring chromosomes and rDNA magnification in Drosophila. Genetics 108: 969-983. GATTI, M. and S. PIMPINELLI, 1983 Cytological and genetic analysis of the Y chromosome of Drosophila melanogaster I. Organization of the fertility factors. Chromosoma 88: 349-373. GATTI,M.,S. PIMPINELLI and G. SANTINI, 1976 Characterization of Drosophila heterochromatin I. Staining and decondensation with Hoechst 33258 and quinacrine. Chromosoma 57: 351375. GRAZIANI, F., R. CAIZZIand S. GARGANO, 1977 Circular ribosomal DNA during ribosomal magnification in Drosophila melanogaster. J. Mol. Biol. 112: 49-63. HAWLEY, R. S . and K. D. TARTOF,1985 A two-stage model for the control of rDNA magnification. Genetics 1 0 9 691-700. HENDERSON, A. and F. RITOSSA,1970 On the inheritance of rDNA of magnified bobbed loci in D. melanogaster. Genetics 66: 463-473. LOCKER,D., 1976 Instability at the bobbed locus following magnification in Drosophila melanogaster. Mol. Gen. Genet. 143: 261-268. LOCKER, D. and M. MARRAKECHI, 1977 Evidence for an excess of rDNA in the testis of Drosophila melanogaster during rDNA magnification. Mol. Gen. Genet. 1 5 4 249-254. BOEEED LETHAL MAGNIFICATION 523 LONG,E. 0. and I. B. DAWID,1979 Restriction analysis of spacers in ribosomal DNA of Drosophila melanogaster. Nucleic Acids Res. 7: 205-215. MANIATIS, T., E. F. FRITSCH, and J. SAMBROOK, 1982 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. MCCLINTOCK, B., 1938 The production of homozygous deficient tissues with mutant characteristics by means of the aberrant mitotic behavior of ring-shaped chromosomes. Genetics 23: 315-376. MCCLINTOCK, B., 1941 The association of mutants with homozygous deficiencies in Zea mays. Genetics 2 6 542-571. MULLER, H. J.. 1932 Further studies on the nature and causes of gene mutations. Proc. 6th Int. Congr. Genet. 1: 213-255. MULLER, H. J., 1948 The construction of several new types of Y chromosomes. Drosophila Inform. Serv. 22: 73-74. NOVITSKI,E., 1952 The genetic consequences of anaphase bridge formation in Drosophila. Genetics 37: 270-287. RITOSSA,F. M., 1968 Unstable redundancy of genes for ribosomal RNA. Proc. Natl. Acad. Sci. USA 6 0 509-516. R"A, F. M., 1972 Procedure for magnification of lethal deletions of genes for ribosomal RNA. Nature (New Biol.) 2 4 0 109-1 1 1 . RITOSSA,F. M., 1973 Crossing over between X and Y chromosomes during ribosomal DNA magnification in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 7 0 1950-1954. RITOSSA, F. M., K. C. ATWOODand S. SPIEGELMAN, 1966 A molecular explanation of the bobbed mutants of Drosophila as partial deficiencies of "ribosomal" DNA. Genetics 54: 819-834. RITOSSA,F. M., C. MALVA,E. BONCINELLI, F. GRAZIANI and L. POLITO,1971 The first steps of magnification of DNA complementary to ribosomal RNA in Drosophila melanogaster. Proc. Nat. Acad. Sci. USA 68: 1580-1584. SANDLER, L., 1954 Heterochromatic exchange between reversed acrocentric compound X chromosomes and FR2. Drosophila Inform. Serv. 28: 153-154. SMITH,G. P., 1973 Unequal crossover and the evolution of multigene families. Cold Spring Harbor Symp. Quant. Biol. 3 8 507-513. SPEAR,B. B., 1974 The genes for ribosomal RNA in diploid and polytene chromosomes of Drosophila melanogaster. Chromosoma 4 8 159- 179. SZOSTAK, J. W. and R. Wu, 1980 Unequal crossing over in the ribosomal DNA of Saccharomyces cereuisiae. Nature 284: 426-430. TARTOF,K. D., 1973a Regulation of ribosomal RNA gene multiplicity in Drosophila melanogaster. Genetics 73: 57-71. TARTOF,K. D., 1973b Unequal mitotic sister chromatid exchange and disproportionate replication as mechanisms regulating ribosomal RNA gene redundancy. Cold Spring Harbor Symp. Quant. Biol. 3 8 491-500. TARTOF,K. D., 1974 Unequal mitotic sister chromatid exchange as the mechanism of ribosomal RNA gene magnification. Proc. Natl. Acad. Sci. USA 71: 1272-1276. Communicating editor: A. SPRADLINC