* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download highlighted topics - American Journal of Physiology
Survey
Document related concepts
Neural oscillation wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neurogenomics wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Central pattern generator wikipedia , lookup
Metastability in the brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Neuroanatomy wikipedia , lookup
Hypothalamus wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Circumventricular organs wikipedia , lookup
Colin Pittendrigh wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Transcript
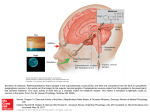
J Appl Physiol 92: 401–408, 2002; 10.1152/japplphysiol.00836.2001. highlighted topics Functional Genomics of Sleep and Circadian Rhythm Invited Review: A neural clockwork for encoding circadian time ERIK D. HERZOG1 AND WILLIAM J. SCHWARTZ2 Department of Biology, Washington University, St. Louis, Missouri 63130; and 2Department of Neurology, University of Massachusetts Medical School, Worcester, Massachusetts 01655 1 suprachiasmatic nucleus; period; oscillator; pacemaker; photoperiod THE EARTH’S DAILY ROTATION about its axis has imposed potent selective pressures on organisms. The fundamental adaptation to the environmental day-night cycle is an endogenous 24-h clock that regulates biological processes in the temporal domain. This clock coordinates physiological events around local (geophysical) time, optimizing the economy of biological systems and allowing for a predictive, rather than purely reactive, homeostatic control. Circadian clocks contribute to the regulation of sleep and reproductive rhythms, seasonal behaviors, and celestial navigation. The practical importance of human circadian rhythmicity, as well as its consequences for health and disease, is now being realized. INVESTIGATING CIRCADIAN RHYTHMICITY AND LOCALIZING A PACEMAKER TO MAMMALIAN BRAIN Many biological activities are restricted to specific times of day. In the absence of external timing cues, Address for reprint requests and other correspondence: W. J. Schwartz, Dept. of Neurology, Univ. of Massachusetts Medical School, 55 Lake Ave. North, Worcester, MA 01655 (E-mail: [email protected]). http://www.jap.org some of these processes remain rhythmic (“free run”) with ⬃24-h (circadian) periods. The features of these self-sustaining oscillations have suggested the existence of an endogenous timekeeping mechanism. The circadian pacemaker receives input (afferent) pathways for synchronization (entrainment) to light-dark cycles and expresses its rhythmicity through output (efferent) pathways. The pacemaker works as a clock because its endogenous period is adjusted to the external 24-h period, primarily by light-induced phase shifts that reset the pacemaker’s oscillation. Advances or delays occur because the pacemaker is differentially sensitive to light exposure at different times in its free-running circadian cycle; this rhythm of light sensitivity can be quantified as a “phase-response curve” (PRC). Variations in photic sensitivity, in concert with changes in the pacemaker’s endogenous period and amplitude, can dramatically affect the temporal sequencing of clock-controlled events. In mammals, a circadian pacemaker has been localized to the suprachiasmatic nucleus (SCN). The body of evidence identifying the SCN as the master circadian pacemaker in mammals is so multidisciplinary in na- 8750-7587/02 $5.00 Copyright © 2002 the American Physiological Society 401 Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 Herzog, Erik D., and William J. Schwartz. Invited Review: A neural clockwork for encoding circadian time. J Appl Physiol 92: 401–408, 2002; 10.1152/japplphysiol.00836.2001.—Many daily biological rhythms are governed by an innate timekeeping mechanism or clock. Endogenous, temperature-compensated circadian clocks have been localized to discrete sites within the nervous systems of a number of organisms. In mammals, the master circadian pacemaker is the bilaterally paired suprachiasmatic nucleus (SCN) in the anterior hypothalamus. The SCN is composed of multiple single cell oscillators that must synchronize to each other and the environmental light schedule. Other tissues, including those outside the nervous system, have also been shown to express autonomous circadian periodicities. This review examines 1) how intracellular regulatory molecules function in the oscillatory mechanism and in its entrainment to environmental cycles; 2) how individual SCN cells interact to create an integrated tissue pacemaker with coherent metabolic, electrical, and secretory rhythms; and 3) how such clock outputs are converted into temporal programs for the whole organism. 402 INVITED REVIEW ASSAYING SCN CIRCADIAN RHYTHMICITY To begin to understand the clockwork’s mechanisms, reliable indicators are needed as markers for the presence, phase, and period of the circadian pacemaker’s oscillation. The use of a variety of observable clockcontrolled outputs allows greater insights. For example, a manipulation that eliminates an overt rhythm might act by inactivating the pacemaker or, equally J Appl Physiol • VOL likely, by uncoupling the measured output from the still-oscillating pacemaker (loss of the clock’s “hands” rather than damage to its “gears”). Given that monitoring the entire pacemaking mechanism is currently impossible, distinguishing between an arrested or uncoupled clock is best assayed from multiple outputs. On the other hand, alterations in the free-running period of a rhythm must reflect changes in pacemaker behavior, either directly or indirectly via an input pathway. Neuronal firing rate. Circadian rhythms of single- or multiple-unit electrical activities in the SCN have been recorded in vivo and in vitro in hypothalamic slices, slice cultures, and dissociated cell cultures (see Ref. 98 for further references). In nocturnal and diurnal rodents, the firing rate is high during the subjective day and low during the subjective night. Recording electrical activity has been an extremely useful assay of SCN rhythmicity, especially for in vitro studies, but it is important to note that Na⫹-dependent action potentials are not a part of the pacemaker’s actual oscillatory apparatus; the internal timekeeping mechanism continues to run unperturbed in the presence of tetrodotoxin, which blocks these action potentials (75, 88). Because electrical discharge represents one of the pacemaker’s outputs and because the nature of the coupling between the circadian pacemaker and firing rate is not known, there may be some experimental conditions in which the patterns of SCN electrical activity might not reflect the state of the pacemaker’s oscillation. Importantly, it is not known how firing rate relates to the coding of efferent signals sent by the SCN to other brain regions. Energy metabolism. SCN metabolic activity was the first property of the nucleus reported to exhibit circadian rhythmicity (72). These experiments utilized an autoradiographic method for the in vivo determination of the rates of glucose utilization of individual structures within the brain by using tracer amounts of 2-deoxy-D-[1-14C]glucose. This assay is useful because regional brain functional activity is closely coupled to regional brain energy utilization, which is dependent on the continuous provision of glucose (80). Like electrical activity, glucose utilization is high during the subjective day and low during the subjective night in both nocturnal and diurnal animals, and the rhythm persists in vitro. The deoxyglucose method has been a helpful assay of SCN rhythmicity in vivo, e.g., in fetal animals, but it too has limitations. A measured level of glucose utilization is not a unitary, indivisible quantity but a dynamic summation of all the individual metabolic costs entailed by an assortment of cellular tasks. Autoradiographic images fail to reflect this underlying complexity, and the spatial resolution of the technique ordinarily does not extend to the level of individual cells. Neuropeptide levels. Rhythms of neuroactive peptides synthesized in the SCN have been measured in the cerebrospinal fluid and in tissues (32). The circadian rhythm of AVP levels has been extensively studied. Levels in the cerebrospinal fluid are highest during the subjective day of both nocturnal and diurnal ani- 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 ture that the strength of this functional localization is unsurpassed by that of any other structure in the vertebrate brain (40). The SCN also appears to function as a seasonal clock underlying photoperiodic time measurement (74). SCN neurons (about 16,000/paired nucleus) are among the smallest in the brain and are very densely packed. In the rat, as a result of a combination of various anatomic techniques, the nucleus is shown to be composed of distinct dorsomedial (“shell”) and ventrolateral (“core”) subdivisions (44). Arginine vasopressin (AVP) or somatostatin is synthesized within some of the dorsal neurons, whereas some ventral neurons synthesize vasoactive intestinal polypeptide (VIP) or gastrin-releasing peptide and are innervated by serotonergic and visual inputs. In Syrian hamsters, a cluster of cells expressing calbindin seems to be crucial for the expression of locomotor rhythmicity (45). Most SCN axons terminate locally within the SCN amid myriad synaptic interactions. ␥-Aminobutyric acid (GABA) appears to be the most plentiful substance and is probably a neurotransmitter in all SCN neurons (55). Over the past few years, analyses of induced and spontaneous mutations, gene sequence homologies, and protein-protein interactions have identified molecular processes within SCN cells that are likely to constitute the actual oscillatory mechanism of the pacemaker (50, 67). It is believed that genes at the clock’s core are functioning as autoregulatory feedback loops, with oscillating levels of nuclear proteins negatively regulating the transcription of their own mRNAs. To date, mutations in the Doubletime (or tau), BMAL1 (or MOP3), Clock, Per1, Per2, and the two Cryptochrome genes have been shown to alter or abolish mammalian circadian rhythmicity [see Albrecht (1a)]. Implicit in the emerging molecular models is the assumption that individual SCN cells contain their own circadian oscillators. Cell autonomous clocks have been definitively demonstrated in fully isolated invertebrate neurons (53) and implicated in cells from the retinas of amphibians and birds (7) and the pineals of birds, reptiles, and fish (16). SCN neurons, when cultured at low density, express firing rate rhythms with different periods on multielectrode arrays (1, 26, 27, 46, 48, 88). This result shows that the SCN is a multioscillator system and strongly suggests that individual SCN cells can act as competent circadian pacemakers. Furthermore, SCN neurons from Doubletime (48) and Clock (26) mutant animals display altered periodicities, supporting a role for these molecules in an intracellular timekeeping mechanism. 403 INVITED REVIEW TRANSLATING PRCS TO THE PHYSIOLOGY OF PACEMAKER INPUTS Because the 24-h alternation of light and darkness is the most obvious indication of the Earth’s daily rotation, it is not surprising that evolution has selected J Appl Physiol • VOL ambient light intensity as the preeminent signal for entraining circadian rhythms to local geophysical time. In mammals, the retina is required for photic entrainment, but the responsible visual system is anatomically and physiologically distinct from the visual systems for oculomotor function and image formation (56). The “circadian” system includes a specialized photoreceptive mechanism that may rely on a novel nonrod, noncone opsin; a subset of ganglion cells that forms a monosynaptic pathway to the SCN [retinohypothalamic tract (RHT)]; and a population of SCN neurons that appears to function as “luminance” detectors, with electrophysiological responses to light that are sustained, proportional to light intensity, and elicited from large receptive fields lacking a retinotopic organization (99). These unique features account for the preserved circadian responses of some apparently “sightless” animals, including the blind mole rat, retinally degenerate mouse mutants, and even some blind people who lack pupillary light reflexes and conscious light perception. Multiple lines of evidence suggest that the excitatory amino acid glutamate is the primary RHT neurotransmitter responsible for mediating the circadian actions of light, with NMDA, AMPA/kainate, and metabotropic receptors all seeming to play some role in mediating glutamate’s postsynaptic effects. Intracellular elevation of both Ca2⫹ and cAMP leads to the phosphorylation of the Ca2⫹/cAMP response-element binding protein (CREB, a transcription factor), and, in the SCN, CREB becomes phosphorylated after photic or glutamatergic stimulation during the subjective night (but not during the subjective day). These data have raised the working hypothesis that light induces a cascade involving the release of glutamate, stimulation of ionotropic receptors, influx of Ca2⫹, activation of nitric oxide synthase and serine/threonine protein kinases, phosphorylation of CREB, and regulation of target gene transcription, e.g., fos, jun, and per (18, 97). As shown at least for c-fos, the threshold, magnitude, and phase dependence of gene activation correlate with light-induced phase shifts of overt circadian rhythmicity. For some of these steps, however, there is a paucity of data on their relationship to one another or to phase shifting. Moreover, at least in rats and hamsters (where SCN subdivisions are clearly defined), the photic and circadian regulation of fos, jun, and per genes appears to occur in separate cell populations (20, 73, 97), implying that the function of these genes may also be cell specific. Further complications to the simplicity of an idealized linear input pathway have been discovered. There is evidence that light-induced phase advances and delays may be mediated by different sets of neurotransmitters and second-messenger networks (18). The pacemaker’s photic input appears to be gated by a rhythm of visual sensitivity in the eyes and modulated by the secretion of RHT peptides (substance P, pituitary adenylate cyclase-activating peptide; Refs. 21, 22) and of serotonin from the midbrain raphe (5-HT1B; Refs. 61, 64). Finally, nonphotic stimuli (e.g., benzodi- 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 mals, and the rhythm persists in hypothalamic explants and dissociated cell cultures. Identifying such peptide rhythms is an important step in analyzing SCN rhythmicity because they provide windows on clock-controlled gene expression (transcription and protein translation and degradation). If the biochemistry of a circadian-regulated process is known, then the molecules involved in its control should have a clear relationship to the oscillatory mechanism of the pacemaker itself. The demonstration that two peptide rhythms can be simultaneously measured in slice cultures (AVP and VIP) suggests the potential power of this approach (77). Transcript levels. Within the SCN, AVP mRNA and peptide levels exhibit circadian rhythmicity, and nuclear run-on analysis has demonstrated that mRNA abundance is regulated by transcription (9). Circadian regulation of the AVP gene is likely mediated by the binding of two transcription factors, CLOCK and BMAL1, to an E box within its promoter (35). Posttranscriptional mechanisms may also contribute to the temporal pattern of SCN AVP expression (8, 69). Another clock-controlled gene within the SCN encodes the bZIP transcription factor albumin D-element binding protein (DBP). Like AVP, its transcription is regulated by the binding of CLOCK to an E-box enhancer (68, 95). Animals lacking AVP or DBP exhibit electrophysiological or behavioral circadian rhythms of decreased amplitude (5, 17, 31). It is clear that a significant percentage of the genome is modulated by the circadian clock. In plants and flies, an estimated ⬃6% of transcripts oscillate on a circadian basis (24, 85). Such a survey has recently been reported for the mouse liver, where roughly 3% of the screened genes were modulated on a circadian basis (41). Several laboratories have now taken advantage of rhythms in transcription to introduce reporter gene constructs for real-time monitoring of gene activity (42, 94, 96). Transgenic SCN carrying the promoter for mouse Per1 linked to the gene for luciferase or enhanced green fluorescent protein (GFP) show robust circadian rhythms in light emission in vitro and in vivo. Because the Per1 gene is ubiquitously expressed in the body, these constructs have been used to test for circadian regulation of Per1 in cultured tissues. Isolated explants from the lung, liver, and kidney have been shown to express a circadian rhythm in Per-driven bioluminescence. The kinetics of such reporters allow for monitoring changes in gene activity that are slower than ⬃1.5 h. Fluorescence from the enhanced GFP reporter is bright enough to be measured in individual cells but requires repeated illumination with potentially damaging ultraviolet radiation. Bioluminescence from luciferase is much dimmer and requires delivery of substrate (luciferin) to the region under study. 404 INVITED REVIEW azepines, cage changes, social interactions) generate a PRC essentially opposite (180° out of phase) to the photic PRC (57). Nonphotic effects appear to be strongly species specific, and the critical physiological variable responsible for their mediation remains unidentified. Elucidating the substrates of nonphotic input pathway(s) should help to clarify pacemaker mechanisms, especially because nonphotic stimuli depress the normally high level of SCN Per1 expressed during the subjective day (29, 51). Much research has focused on the likely roles of serotonin-containing (5-HT1A/7) and neuropeptide Y-containing afferents to the SCN (the latter from the intergeniculate leaflet of the thalamus) (54). ASSEMBLING A MULTICELLULAR CIRCADIAN PACEMAKER IN THE SCN J Appl Physiol • VOL CONVERTING MULTIPLE PACEMAKER OUTPUT MECHANISMS INTO TEMPORAL PROGRAMS The SCN governs a wide array of rhythms, from biosynthetic to behavioral, exhibiting a range of waveforms and phases different from the central oscillation that drives them. Because the pacemaker’s outputs are coupled to effector cells via synaptic transmission and hormonal secretion and indirectly through the rhythmic regulation of behavior, the fidelity of the circadian signal might be altered and overt rhythms could differ substantially from their underlying cellular and molecular oscillations. The timing and waveforms of manifest rhythms are also shaped by noncircadian factors, as exemplified by the regulation of sleep (in which consolidated sleep results from the nonadditive interaction of a circadian process with a homeostatic factor related to the duration of preceding wakefulness) (14a, 15, 92). Further complicating the conceptual and experimental analysis of this problem is the existence of nested feedback loops, by which some of the pacemaker’s rhythmic outputs may modulate the behavior of the pacemaker itself. As examples, rhythmic locomotion on a running wheel appears to modify the pacemaker’s free-running circadian period, whereas pineal melatonin secretion (also driven by the SCN) has phase-resetting and entraining effects on overt rhythmicity via melatonin receptors on SCN neurons (30, 47). Of the multisynaptic pathways from the SCN to distal targets, the best studied is the one regulating pineal melatonin synthesis. The paraventricular nucleus of the hypothalamus receives extensive SCN projections, and the neurotransmitter GABA may form a necessary link in this pathway (36, 37). The circuit is completed by paraventricular nucleus projections to preganglionic neurons of the sympathetic nervous sys- 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 In vivo and in cultured explants, SCN cells synchronize their circadian rhythms to one another. The genotype-specific free-running period characteristically expressed by whole animals may represent the mean period arising from the coupling of these multiple SCN cellular oscillators (26, 27, 48, 49); this contrasts with many other rhythmic tissues (e.g., the heart), in which the faster cells set the rate. The SCN’s intercellular coupling mechanism is unknown. Interest has focused on GABA (19, 46, 86), nonsynaptic/dendritic exocytosis (10), and chemical and electrical low-resistance interneuronal junctions (11, 28, 34, 76, 78). Glial cells are also likely to be an important part of any synchronizing mechanism; intercellular Ca2⫹ waves can travel long distances across cultured SCN astrocytes, presumably due to gap junctions (84, 89). Based initially on behavioral data, the rodent circadian clock has been modeled as a complex pacemaker consisting of two mutually coupled oscillators (62). Variability in the phase relationship between these two oscillators could account for several circadian properties, including the ability to measure seasonal changes of day length, with a morning oscillator (M) accelerated by light and synchronized to dawn, and an evening oscillator (E) decelerated by light and synchronized to dusk. There is evidence to suggest that the circadian system is functionally organized into two such oscillating components. Light pulses have been reported to cause independent phase shifts of the evening onset and morning offset of two circadian rhythms dependent on the SCN, one biochemical [pineal N-acetyltransferase activity (NAT)] and the other behavioral (locomotion) (for references, see Refs. 12 and 74). Recordings from cultured SCN explants have demonstrated that the vast majority of neurons reach their peak of firing together, with a smaller population that is ⬃12 h out of phase (25, 58). Recording of Per1-driven GFP fluorescence has shown similar results but with cells assuming perhaps more phase relationships (65). Recently, dual circadian oscillations have been demonstrated in SCN tissue isolated in vitro (33). In slices from Syrian hamsters, the circadian rhythm of SCN multiunit neuronal activity exhibits distinct morning and evening peaks when the slices are cut in the horizontal plane. The morning peak follows projected dawn, whereas the evening peak occurs around projected dusk. The two peaks are differentially affected by changes in the antecedent photoperiod, and they are shifted independently in a phasedependent manner after stimulation with glutamate. Assuming that M and E oscillators really do exist, it is not known whether they are a property of individual SCN cells or instead emerge from an intercellular (network) interaction within SCN tissue. The latter mechanism accounts for the phenomenon known as “splitting” (seen especially, but not exclusively, in Syrian hamsters maintained in constant light), in which an animal’s single daily bout of locomotor activity dissociates into two components that each free run with different periods until they become stably coupled at 180° (⬃12 h) apart. This behavior appears to be a consequence of a paired SCN that has become reorganized into two oppositely phased, left- and right-sided circadian pacemakers (14). However, most current data suggest that these “split” oscillators are unlikely to represent M and E. 405 INVITED REVIEW J Appl Physiol • VOL Fig. 1. Model of the organization of the mammalian circadian system from the master pacemaker [suprachiasmatic nucleus (SCN)] to its various targets in the brain and body. The SCN, in the hypothalamus, contains cells that are competent, sustained circadian oscillators. In vivo, they are synchronized to one another, with the ensemble showing maximum electrical and metabolic activity during the day. Efferent pathways, both neural and humoral, terminate on structures that are either weakly rhythmic (bottom) or arrhythmic (top). The arrhythmic structures most likely receive inhibitory input from the SCN and are thus driven to produce rhythms in anti-phase to the SCN. The weakly rhythmic structures studied thus far have all shown activity that is high during the night. It is not clear whether their damped rhythmicity is intrinsic to single cells or the result of rhythmic cells drifting out of phase from one another. This multioscillator system can drive rhythms at various phases and with different amplitudes, providing a temporal control of physiology and behavior that anticipates daily events and adapts to changes in environmental cycles. (with an ⬃100-fold nocturnal increase in rat) and, with the recent cloning of the NAT gene, the best understood biochemical rhythm in mammals (39). In rodents, a mechanism involving protein kinase A and the phosphorylation of CREB induces NAT gene transcription and the consequent elevation of mRNA content, protein level, and enzymatic activity. On the other hand, a posttranscriptional mechanism, proteosomal proteolysis, appears to be the dominant NAT regulatory mechanism in ungulates, in which a modest nocturnal increase of NAT mRNA levels (⬃1.5-fold) does not account for the ⬃10-fold increase of NAT enzymatic activity. Another well-analyzed cellular rhythm is in the rat liver, in which DBP mRNA and protein levels exhibit a circadian rhythm, with a ⬃100-fold magnitude protein oscillation that peaks ⬃2 h after lights off in a light-dark cycle (91). Rhythmic DBP levels appear responsible for circadian transcription of the cholesterol 7-␣-hydroxylase gene (43), which encodes the rate-limiting enzyme for the conversion of cholesterol to bile acids [maximal during the dark (feeding) phase in nocturnal rats]. CODA The circadian timekeeping system is a unique and powerful model for investigating the cellular and molecular mechanisms that underlie behavioral state con- 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 tem (in the intermediolateral column of the spinal cord) and postganglionic cell bodies in the superior cervical ganglion that noradrenergically synapse in the pineal gland. Other SCN efferent fibers contain AVP or VIP and have been implicated in the circadian regulation of reproductive (59, 60) and adrenal (6, 38) function. On the other hand, the rhythm of locomotor activity appears to depend on an SCN-secreted, diffusible substance; locomotor rhythmicity can be reestablished in SCN-lesioned hamsters by transplants of SCN tissue isolated within semipermeable polymeric capsules that prevent neural outgrowth but allow diffusion of substances with mass ⬍500 kDa (79). The soluble signal released by the encapsulated tissue is unknown. Although extra-SCN oscillators have been suspected in mammals for some time, a direct demonstration was first provided by measuring a circadian rhythm of melatonin synthesis in hamster retinas in vitro (82). Since then, a variety of cultured mammalian tissues (96) and even immortalized fibroblasts (3, 93) have been shown to exhibit damped oscillations of “clock” gene expression. The sustained cycling of these peripheral oscillators probably depends on the SCN (71), and ongoing work is investigating the possible coupling mechanisms between such brain and body rhythms. A phase shift of the light-dark cycle immediately shifts SCN Per1 gene transcription, but peripheral tissues require up to several days to reestablish their normal phase relationships to the SCN and each other (96). On the other hand, restricted feeding entrains circadian oscillations in the liver (13, 23, 81) and in the cerebral cortex and hippocampus (87) but not in the SCN. One surprising finding is that redox state (e.g., the ratio of NAD⫹ to NADH within a cell) can directly affect the activity of circadian transcription factors (70). This raises the exciting possibility that the phase and, perhaps, the generation of circadian rhythms are directly linked to factors that sense the metabolic fluctuations within the cell (66). Both humoral (2, 52) and neural (83) signals are likely to be involved in tissue synchronization, probably via multiple signaling pathways (4). This contrasts with the situation in semitransparent animals (like flies and zebrafish), where the peripheral oscillators appear to be directly photosensitive (63, 90). These results raise important questions about whether some of the transitions seen in circadian behavior following shifts of the light schedule (e.g., with jet lag after transmeridian travel) may be the result of a “supra-SCN” multioscillator organization. Output pathways are thus likely to provide an important substrate for plasticity in the circadian timing system. The SCN may not only drive, but also entrain, rhythmicity in downstream structures (Fig. 1). The functional implications and adaptive significance of these organismal differences in circadian organization should be an exciting area for future investigation. Ultimately, clock regulation of physiology and behavior must rely in some way on changes in cellular biochemistry. The circadian rhythm of the enzymatic activity of pineal NAT is arguably the most robust 406 INVITED REVIEW trol. A natural stimulus (light) can be presented in a controlled fashion, resulting in a long-lasting neural change (in the pacemaker’s oscillation), which can be measured as an adaptive behavioral response (a permanent phase shift of overt rhythmicity). As a result, there are remarkable opportunities to begin to understand behavior at multiple levels of biological organization, ranging from intracellular regulatory molecules and gene expression, to intercellular networks and multisynaptic pathways, and finally to integrated patterns of physiology and performance. 18. 19. 20. 21. REFERENCES J Appl Physiol • VOL 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 1. Abe M, Herzog ED, and Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport 11: 3261–3264, 2000. 1a.Albrecht U. Invited Review: Regulation of mammalian circadian clock genes. J Appl Physiol In press. 2. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, and Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289: 2344–2347, 2000. 3. Balsalobre A, Damiola F, and Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937, 1998. 4. Balsalobre A, Marcacci L, and Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 10: 1291–1294, 2000. 5. Brown MH and Nunez AA. Vasopressin-deficient rats show a reduced amplitude of the circadian sleep rhythm. Physiol Behav 46: 759–762, 1989. 6. Buijs RM, Wortel J, van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, and Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11: 1535– 1544, 1999. 7. Cahill GM and Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Ret Eye Res 14: 267–291, 1995. 8. Carter DA and Murphy D. Diurnal rhythm of vasopressin mRNA species in the rat suprachiasmatic nucleus: independence of neuroendocrine modulation and maintenance in explant culture. Brain Res 6: 233–239, 1989. 9. Carter DA and Murphy D. Nuclear mechanisms mediate rhythmic changes in vasopressin mRNA expression in the rat suprachiasmatic nucleus. Brain Res 12: 315–321, 1992. 10. Castel M, Morris J, and Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. Neuroreport 7: 543–547, 1996. 11. Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J Neurobiol 43: 379–388, 2000. 12. Daan S, Albrecht U, van der Horst GT, Illnerová H, Roenneberg T, Wehr TA, and Schwartz WJ. Assembling a clock for all seasons: are there M and E oscillators in the genes? J Biol Rhythms 16: 105–116, 2001. 13. Damiola F, Le Minh N, Preitner N, Kornmann B, FleuryOlela F, and Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14: 2950–2961, 2000. 14. De la Iglesia HO, Meyer J, Carpino A, and Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290: 799–801, 2000. 14a.Dijk D-J and Lockley SW. Invited Review: Human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. In press. 15. Edgar DM, Dement WC, and Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci 13: 1065–1079, 1993. 16. Falcón J. Cellular circadian clocks in the pineal. Prog Neurobiol 58: 121–162, 1999. 17. Franken P, Lopez-Molina L, Marcacci L, Schibler U, and Tafti M. The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. J Neurosci 20: 617– 625, 2000. Gillette MU and Tischkau SA. Suprachiasmatic nucleus: the brain’s circadian clock. Recent Prog Horm Res 54: 33–58, 1999. Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, De Jeu MT, Pennartz CM, and Dudek FE. A reexamination of the role of GABA in the mammalian suprachiasmatic nucleus. J Biol Rhythms 14: 126–130, 1999. Hamada T, LeSauter J, Venuti JM, and Silver R. Expression of Period genes: rhythmic and non-rhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci 19: 7742– 7750, 2001. Hamada T, Yamanouchi S, Watanabe A, Shibata S, and Watanabe S. Involvement of glutamate release in substance P-induced phase delays of suprachiasmatic neuron activity rhythm in vitro. Brain Res 836: 190–193, 1999. Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, and Mikkelsen JD. Pituitary adenylate cylcaseactivating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci 17: 2637–2644, 1997. Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, and Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6: 269–278, 2001. Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, and Kay SA. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113, 2000. Herzog ED, Geusz ME, Khalsa SB, Straume M, and Block GD. Circadian rhythms in mouse suprachiasmatic nucleus explants on multimicroelectrode plates. Brain Res 757: 285–290, 1997. Herzog ED, Takahashi JS, and Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci 1: 708–713, 1998. Honma S, Shirakawa T, Katsuno Y, Namihira M, and Honma K. Circadian periods of single suprachiasmatic neurons in rats. Neurosci Lett 250: 157–160, 1998. Honma S, Shirakawa T, Nakamura W, and Honma K. Synaptic communication of cellular oscillations in the rat suprachiasmatic neurons. Neurosci Lett 294: 113–116, 2000. Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya T, Okamura H, and Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J Neurosci 20: 5867–5873, 2000. Hunt AE, Al Ghoul WM, Gillette MU, and Dubocovich ML. Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol 280: C110–C118, 2001. Ingram CD, Snowball RK, and Mihai R. Circadian rhythm of neuronal activity in suprachiasmatic nucleus slices from the vasopressin-deficient Brattleboro rat. Neuroscience 75: 635–641, 1996. Inouye ST and Shibata S. Neurochemical organization of circadian rhythm in the suprachiasmatic nucleus. Neurosci Res 20: 109–130, 1994. Jagota A, de la Iglesia HO, and Schwartz WJ. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci 3: 372–376, 2000. Jiang ZG, Yang YQ, and Allen CN. Tracer and electrical coupling of rat suprachiasmatic nucleus neurons. Neuroscience 77: 1059–1066, 1997. Jin X, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, and Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96: 57–68, 1999. Kalsbeek A, Cutrera RA, van Heerikhuize JJ, van der Vliet J, and Buijs RM. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience 91: 453– 461, 1999. 407 INVITED REVIEW J Appl Physiol • VOL 59. Palm IF, Der Beek EM, Swarts HJ, Vliet J, Wiegant VM, Buijs RM, and Kalsbeek A. Control of the estradiol-induced prolactin surge by the suprachiasmatic nucleus. Endocrinology 142: 2296–2302, 2001. 60. Palm IF, van der Beek EM, Wiegant VM, Buijs RM, and Kalsbeek A. The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res 901: 109–116, 2001. 61. Pickard GE, Smith BN, Belenky M, Rea MA, Dudek FE, and Sollars PJ. 5-HT1B receptor-mediated presynaptic inhibition of retinal input to the suprachiasmatic nucleus. J Neurosci 19: 4034–4045, 1999. 62. Pittendrigh CS and Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol [A] 106: 333–355, 1976. 63. Plautz JD, Kaneko M, Hall JC, and Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science 278: 1632–1635, 1997. 64. Quintero JE and McMahon DG. Serotonin modulates glutamate responses in isolated suprachiasmatic nucleus neurons. J Neurophysiol 82: 533–539, 1999. 65. Quintero JE, Speck DF, and McMahon DG. Monitoring mouse Period1-driven green fluorescent protein gene expression in slices and in individual cells from the biological clock (Abstract). Soc Neurosci Abstr 26: 205, 2000. 66. Reick M, Garcia JA, Dudley C, and McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science 293: 506–509, 2001. 67. Reppert SM and Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63: 647–676, 2001. 68. Ripperger JA, Shearman LP, Reppert SM, and Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev 14: 679– 689, 2000. 69. Robinson BG, Frim DM, Schwartz WJ, and Majzoub JA. Vasopressin mRNA in the suprachiasmatic nuclei: daily regulation of polyadenylate tail length. Science 241: 342–344, 1988. 70. Rutter J, Reick M, Wu LC, and McKnight SL. Regulation of Clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293: 510–514, 2001. 71. Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, and Ishida N. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem 273: 27039– 27042, 1998. 72. Schwartz WJ. SCN metabolic activity in vivo. In: Suprachiasmatic Nucleus: The Mind’s Clock, edited by Klein DC, Moore RY, and Reppert SM. New York: Oxford Univ. Press, 1991, p. 144– 156. 73. Schwartz WJ, Carpino A, de la Iglesia HO, Baler R, Klein DC, Nakabeppu Y, and Aronin N. Differential regulation of fos family genes in the ventrolateral and dorsomedial subdivisions of the rat suprachiasmatic nucleus. Neuroscience 98: 535– 547, 2000. 74. Schwartz WJ, de la Iglesia HO, Zlomanczuk P, and Illnerová H. Encoding Le Quattro Stagioni within the mammalian brain: photoperiodic orchestration through the suprachiasmatic nucleus. J Biol Rhythms 16: 302–311, 2001. 75. Schwartz WJ, Gross RA, and Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA 84: 1694–1698, 1987. 76. Shinohara K, Funabashi T, Mitushima D, and Kimura F. Effects of gap junction blocker on vasopressin and vasoactive intestinal polypeptide rhythms in the rat suprachiasmatic nucleus in vitro. Neurosci Res 38: 43–47, 2000. 77. Shinohara K, Honma S, Katsuno Y, Abe H, and Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA 92: 7396–7400, 1995. 78. Shirakawa T, Honma S, Katsuno Y, Oguchi H, and Honma KI. Synchronization of circadian firing rhythms in cultured rat suprachiasmatic neurons. Eur J Neurosci 12: 2833–2838, 2000. 79. Silver R, LeSauter J, Tresco PA, and Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 37. Kalsbeek A, Garidou ML, Palm IF, van der Vliet J, Simonneaux V, Pevet P, and Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur J Neurosci 12: 3146–3154, 2000. 38. Kalsbeek A, van Heerikhuize JJ, Wortel J, and Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamopituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci 16: 5555–5565, 1996. 39. Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Begay V, Falcon J, Cahill GM, Cassone VM, and Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res 52: 307–357, 1997. 40. Klein DC, Moore RY, and Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford Univ. Press, 1991. 41. Kornmann B, Preitner N, Rifat D, Fleury-Olela F, and Schibler U. Analysis of circadian liver gene expression by ADDER, a highly sensitive method for the display of differentially expressed mRNAs. Nucleic Acids Res 29: E51, 2001. 42. Kuhlman SJ, Quintero JE, and McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport 11: 1479–1482, 2000. 43. Lavery DJ and Schibler U. Circadian transcription of the cholesterol 7␣ hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev 7: 1871–1884, 1993. 44. Leak RK, Card JP, and Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res 819: 23–32, 1999. 45. LeSauter J and Silver R. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci 19: 5574–5585, 1999. 46. Liu C and Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25: 123–128, 2000. 47. Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, and Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19: 91–102, 1997. 48. Liu C, Weaver DR, Strogatz SH, and Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell 91: 855–860, 1997. 49. Low-Zeddies SS and Takahashi JS. Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell 105: 25–42, 2001. 50. Lowrey PL and Takahashi JS. Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet 34: 533–562, 2000. 51. Maywood ES, Mrosovsky N, Field MD, and Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci USA 96: 15211–15216, 1999. 52. McNamara P, Seo S, Rudic RD, Sehgal A, Chakravarti D, and FitzGerald GA. Regulation of clock and mop4 by nuclear hormone receptors in the vasculature. A humoral mechanism to reset a peripheral clock. Cell 105: 877–889, 2001. 53. Michel S, Geusz ME, Zaritsky JJ, and Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science 259: 239–241, 1993. 54. Mistlberger RE and Antle MC. Behavioral inhibition of lightinduced circadian phase resetting is phase and serotonin dependent. Brain Res 786: 31–38, 1998. 55. Moore RY and Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett 150: 112–116, 1993. 56. Morin LP. The circadian visual system. Brain Res Rev 19: 102–127, 1994. 57. Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc 71: 343–372, 1996. 58. Nakamura W, Honma S, Shirakawa T, and Honma KI. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci 14: 1–10, 2001. 408 80. 81. 82. 83. 84. 85. 86. 87. 89. nucleus controlling circadian locomotor rhythms. Nature 382: 810–813, 1996. Sokoloff L. Circulation and energy metabolism of the brain. In: Basic Neurochemistry, edited by Siegel GJ. New York: Raven, 1989, p. 565–590. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, and Menaker M. Entrainment of the circadian clock in the liver by feeding. Science 291: 490–493, 2001. Tosini G. Melatonin circadian rhythm in the retina of mammals. Chronobiol Int 17: 599–612, 2000. Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, and Loewy AD. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 2: 1051–1053, 1999. Van den Pol AN, Finkbeiner SM, and Cornell-Bell AH. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci 12: 2648–2664, 1992. Van Gelder RN, Bae H, Palazzolo MJ, and Krasnow MA. Extent and character of circadian gene expression in Drosophila melanogaster: identification of twenty oscillating mRNAs in the fly head. Curr Biol 5: 1424–1436, 1995. Wagner S, Castel M, Gainer H, and Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387: 598–603, 1997. Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, and Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13: 1190–1196, 2001. Welsh DK, Logothetis DE, Meister M, and Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14: 697–706, 1995. Welsh DK and Reppert SM. Gap junctions couple astrocytes but not neurons in dissociated cultures of rat suprachiasmatic nucleus. Brain Res 706: 30–36, 1996. J Appl Physiol • VOL 90. Whitmore D, Foulkes NS, and Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature 404: 87–91, 2000. 91. Wuarin J and Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell 63: 1257–1266, 1990. 92. Wurts SW and Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci 20: 4300–4310, 2000. 93. Yagita K, Tamanini F, van der Horst GT, and Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science 292: 278–281, 2001. 94. Yamaguchi S, Kobayashi M, Mitsui S, Ishida Y, van der Horst GT, Suzuki M, Shibata S, and Okamura H. View of a mouse clock gene ticking. Nature 409: 684, 2001. 95. Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, and Okamura H. Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol 20: 4773–4781, 2000. 96. Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, and Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000. 97. Yan L, Takekida S, Shigeyoshi Y, and Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience 94: 141–150, 1999. 98. Zlomanczuk P and Schwartz WJ. Cellular and molecular mechanisms of circadian rhythms in mammals. In: Regulation of Sleep and Circadian Rhythms, edited by Turek FW and Zee PC. New York: Dekker, 1999, p. 309–342. 99. Zordan MA, Rosato E, Piccin A, and Foster R. Photic entrainment of the circadian clock: from Drosophila to mammals. Sem Cell Dev Biol 12: 317–328, 2001. 92 • JANUARY 2002 • www.jap.org Downloaded from http://jap.physiology.org/ by 10.220.33.3 on June 16, 2017 88. INVITED REVIEW