* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download HARVESTING CHEMICAL ENERGY: CELLULAR
Signal transduction wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Phosphorylation wikipedia , lookup
Mitochondrion wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biochemistry wikipedia , lookup
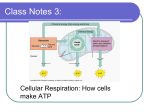
8 HARVESTING CHEMICAL ENERGY: CELLULAR RESPIRATION Chapter Outline 8.1 OVERVIEW OF CELLULAR ENERGY METABOLISM Coupled oxidation and reduction reactions produce the flow of electrons for energy metabolism Electrons flow from fuel substances to final electron acceptors In cellular respiration, cells make ATP by oxidative phosphorylation 8.2 GLYCOLYSIS The reactions of glycolysis include energy-requiring and energy-releasing steps Glycolysis is regulated at key points 8.3 PYRUVATE OXIDATION AND THE CITRIC ACID CYCLE Pyruvate oxidation produces the two-carbon fuel of the citric acid cycle The citric acid cycle oxidizes acetyl groups completely to CO2 Carbohydrates, fats, and proteins can function as electron sources for oxidative pathways 8.4 THE ELECTRON TRANSFER SYSTEM AND OXIDATIVE PHOSPHORYLATION In the electron transfer system, electrons flow through protein complexes in the inner mitochondrial membrane Ubiquinone and the three major electron transfer complexes pump H+ across the inner mitochondrial membrane Chemiosmosis powers ATP synthesis by a proton gradient Thirty-two ATP molecules are produced for each molecule of glucose completely oxidized to CO2 and H2O Cellular respiration conserves more than 30% of the chemical energy of glucose in ATP 8.5 FERMENTATION Fermentation keeps ATP production going when oxygen is unavailable Unanswered Questions Learning Objectives After reading the chapter, you should be able to: 1. Describe the difference between cellular respiration and oxidative phosphorylation. 2. Explain the function of a proton gradient and how it is formed during cellular respiration. Describe how this proton gradient is related to the production of ATP. 3. Discuss the role of ATP synthase in the production of ATP. 4. Explain the differences between cellular respiration and fermentation. 5. List the three major phases of cellular respiration and give a brief description of what is the starting material and what is produced in each phase. 6. Describe briefly the role of redox reactions in the production of ATP from glucose. 7. Describe the two main pathways in which cells produce ATP from glucose and what types of conditions in the cell govern which pathway to take (aerobic vs. anaerobic). 8. Explain the importance of the structure of mitochondria and the location of transport proteins for reactions involved in cellular respiration. Key Terms 68 Chapter Eight Woelker 2009 cellular respiration acetyl-CoA oxidation oxidative phosphorylation FAD, FADH2 chemiosmotic hypothesis oxidized ATP synthase tricarboxylic acid cycle chemiosmosis reduction cell fractionation Krebs cycle brown fat reduced pyruvate, pyruvic acid uncoupling proteins redox reactions NAD , NADH mitochondrial electron transfer system glycolysis glyceraldehyde-3phosphate proton (H ) gradient lactate fermentation cytochrome c alcoholic fermentation citric acid cycle substrate-level phosphorylation ubiquinone strict anaerobes electron transfer system phosphofructokinase cytochromes facultative anaerobes proton-motive force strict aerobes pyruvate oxidation + + fermentation Lecture Outline A. Mitochondria are the powerhouses of our cells. B. Many disease conditions are related to defective mitochondria. 1. In the 1960s, Swedish physician Rolf Luft discovered a condition associated with defective mitochondria that produce less ATP than normal mitochondria (Luft syndrome). 2. Conditions associated with defective mitochondria can be inherited. 3. Defective mitochondria may contribute to other age-related problems including diabetes, atherosclerosis, ALS, and Parkinson, Alzheimer, and Huntington diseases. C. Cellular respiration is the production of cellular energy (i.e., ATP) from the metabolic breakdown of food molecules. 1. ATP fuels nearly all cellular activity in prokaryotes and eukaryotes. 2. Respiration powers most processes in prokaryotes and eukaryotes. D. Photosynthesis, discussed in Chapter 9, is the process of capturing light energy to produce (food) molecules needed by other organisms for cellular respiration. 1. Photosynthesis also produces the oxygen needed for cellular respiration to occur. 2. Cellular respiration could not occur without photosynthesis. 3. Photosynthesis occurs in plants, protists, and some prokaryotes. 4. Cellular respiration and photosynthesis are part of the carbon cycle. 8.1 Overview of Cellular Energy Metabolism A. Electrons are exchanged between molecules during the process of energy production. 1. Electrons are removed from food molecules, particularly sugars, and passed to oxygen during cellular respiration. 2. Some of the energy of the electrons is used in the synthesis of ATP. B. Oxidation is the removal of electrons from a molecule, and reduction is the addition of electrons to a molecule. 1. Oxidation and reduction reactions are coupled in cells and are referred to as redox reactions. 2. Sometimes electron transfer results in the rearrangement of bonds in molecules. a. The redox reaction between methane and oxygen produces carbon dioxide and water. b. This reaction illustrates a change in degree of electron sharing. C. The movement of electrons from an atom requires energy. 1. Highly electronegative atoms hold on to electrons very tightly. 2. Changes in electron positions during a redox reaction result in changes in the chemical energy of the reactants and products. a. When methane is burned in the presence of oxygen, electrons are held more tightly in the products than the reactants. b. Chemical energy is released as a result of the change in energy of the molecules. 3. Electrons move from fuel substances to final electron acceptors. a. The lower the energy of the final electron acceptor, the greater the yield of energy for cellular activities. b. Oxygen is the major electron acceptor in most cells. D. The energy of electrons used during cellular respiration originates in the reactions of photosynthesis. Woelker 2009 Harvesting Chemical Energy: Cellular Respiration 69 1. Electrons are taken from water and used to make sugars during photosynthesis. 2. Light energy is used to move the electrons. 3. Sugars are used during oxidative processes to make ATP during cellular respiration. E. During cellular respiration, cells make ATP by oxidative phosphorylation. 1. Glucose is the major fuel for cellular respiration. 2. Glucose transfers electrons to oxygen forming water during cellular respiration. a. Carbon dioxide and ATP are by-products of this process. b. Phosphorylation results when a phosphate group is transferred to a molecule (such as a phosphate group added to ADP). F. There are three stages of cellular respiration. 1. Glycolysis is the breakdown of glucose to pyruvate with some production of ATP. 2. Pyruvate oxidation is the conversion of pyruvate to acetyl-coA. a. Acetyl-coA is then oxidized completely to carbon dioxide (citric acid cycle). b. Some ATP is produced during the citric acid cycle. 3. Electron transfer is the movement of electrons produced during glycolysis, pyruvate oxidation, and the citric acid cycle from electron carriers to oxygen. a. Water is produced during this process. b. Oxidative phosphorylation uses a hydrogen (H+) gradient produced during electron transport to fuel the production of lots of ATP. G. Most cellular respiration processes in eukaryotes occur in the mitochondria except for glycolysis, which occurs in the cytoplasm of cells. 1. Pyruvate oxidation and the citric acid cycle occur in the mitochondrial matrix. 2. Electron transport and oxidative phosphorylation are associated with proteins/enzymes of the inner mitochondrial matrix. H. Glycolysis, pyruvate oxidation, and the citric acid cycle occur in the cytoplasm of prokaryotes whereas other cellular respiration processes are associated with the plasma membrane. I. Research Method: Cell Fractionation 1. Fractionation techniques are used to separate cellular components such as mitochondria, chloroplasts, or other organelles from one another. a. Sonication, homoginization, detergents, or other techniques are first used to break open cells. b. Centrifugation is used to “spin down” cellular components based on their shape and density at different speeds/gravitational forces. 2. Once isolated, organelles themselves can be “opened” to release their content (i.e., DNA, protein, etc.). a. Mitochondria can be treated to separate the inner and outer membranes. b. The functions of structures in the different mitochondrial membranes can be determined once isolated. 8.2 Glycolysis A. Enzymes in the cytoplasm catalyze the breakdown of a six-carbon sugar (glucose) into two molecules of a three-carbon sugar (pyruvate). 1. Glycolysis results in the production of small amounts of ATP. 2. Glycolysis is also known as the Embden-Meyerhof pathway named after two German physiological chemists. B. Glycolysis begins as energy-requiring reactions. 1. Glucose is first phosphorylated, then split to form two molecules of PGAL, using two ATP molecules. 2. The two molecules of PGAL are ready to enter the next reaction. C. Glycolysis continues as energy-releasing reactions. 1. Enzymes remove H+ and electrons from PGAL to change NAD+ to NADH (which is used later in electron transfer). 2. By substrate-level phosphorylation, four ATPs are produced. D. The end products of glycolysis are (for each glucose molecule): two pyruvates, two ATPs (net gain), and two NADH. E. There are ten steps in the process of converting glucose to pyruvate. 1. The major oxidation occurs during step 6 where two electrons and two protons (H +) are removed from G3P. a. Both electrons and one proton are transferred to NAD+ from NADH. b. One proton is released into the cytoplasm. 70 Chapter Eight Woelker 2009 2. 3. 4. F. Reactions 1–5 generate G3P, using ATP. Reactions 6–10 convert G3P to pyruvate, producing ATP and NADH. The net reaction is: glucose + 2 ADP + 2 Pi + 2 NAD+ → 2 pyruvate + 2 NADH + 2 H+ + 2 ATP a. All six carbons from glucose are retained in the two pyruvate molecules. b. Each ATP molecule is produced during steps 8 and 10, resulting from substrate-level phosphorylation. Glycolysis is regulated at key points. 1. The rate of sugar oxidation is regulated by a cell’s need for energy. a. If enough ATP is present, some binds to the enzymes responsible for glycolysis (e.g., phosphofructokinase) and inhibits the action of the enzyme. b. If ATP levels are low (and ADP levels high), the enzymes responsible for glycolysis are not inhibited and glycolysis proceeds. 2. The presence of certain molecules can affect the enzymes that control glycolysis. a. Excess NADH can bind to and inhibit phosphofructokinase. b. Limited oxygen often results in excess NADH. 8.3 Pyruvate Oxidation and the Citric Acid Cycle A. Pyruvate oxidation produces acetyl-CoA. 1. Pyruvate molecules produced during glycolysis are transported into the mitochondrial matrix and converted into acetyl-CoA. a. A multi-enzyme complex removes one carbon from pyruvate and attaches it to oxygen forming carbon dioxide. b. The two-carbon fragment remaining joins coenzyme A to form acetyl-CoA. 2. Two reactions actually occur since two pyruvate molecules are produced during glycolysis, and a net two NAD+ are reduced to NADH in the process. a. Two electrons and two hydrogen ions are actually produced during the reaction. b. The last proton is released as free H+. B. The citric acid cycle oxidizes acetyl-coA completely and produces CO2. 1. The cycle was named for citrate, the first compound produced during the process. a. The process is also called the tricarboxylic acid cycle or Krebs cycle after the scientist Sir Hans Krebs who worked out the majority of the reactions. b. Other organic acids, including succinate, fumarate, and acetate, are also oxidized in the cycle. 2. The cycle occurs in the mitochondrial matrix and involves eight steps. a. In one turn of the cycle, acetyl-coA is consumed and two molecules of carbon dioxide are produced. b. The coA molecules are recycled in the process and used for further cycles. 3. Three oxidation steps use NAD+ as an electron acceptor producing 3 NADHs, and one uses FAD+ producing FADH2. 4. Substrate-level phosphorylation generates one ATP. 5. Because two molecules of acetyl-coA are produced from one molecule of glucose, the cycle turns twice and twice the products are produced (i.e., 6 NADH, 2 FADH2, and 2 ATP). 6. The cycle is regulated, as is glycolysis, by increased amounts of cycle by-products. a. For example, the first enzyme in the cycle, citrate synthase, is inhibited by high ATP concentrations. b. The cycle typically stops if ATP production exceeds the cell’s demands to conserve cellular fuels, such as carbohydrates. 7. Carbohydrates, fats, and proteins can be used for the citric acid cycle. a. Other disaccharides, such as sucrose, can be broken down into glucose or fructose and shuttled into early stages of the cycle. b. Fatty acids can be converted into glycerol or acetyl-coA and enter the cycle at stages 6 or stage 1, respectively, of the cycle. c. Proteins are hydrolyzed to amino acids and eventually converted into pyruvate, acetyl-coA , or some other intermediate before entering the cycle. 8.4 The Electron Transfer System and Oxidative Phosphorylation A. Electron transfer and phosphorylation 1. Enzyme systems (electron carriers) embedded in the mitochondrial inner membrane remove the electrons from NADH and FADH2 produced during the citric acid cycle. Woelker 2009 Harvesting Chemical Energy: Cellular Respiration 71 a. b. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 72 Chapter Eight The hydrogen ions are transferred to the inner mitochondrial space. The flow of hydrogen ions back through ATP synthases in the inner membrane produce ATP by directly phosphorylating ADP to ATP. The inner mitochondrial carriers occur as protein complexes. a. Three complexes, numbered I, III, and IV, are transmembrane structures of the inner membrane. b. One complex, numbered II, is bound to the inner membrane on the matrix side. Two small, mobile electron carriers, cytochrome c and ubiquinone (also known as coenzyme Q or CoQ), shuttle electrons. Complex I electron carriers pick up electrons from NADH. Complex II electron carriers pick up electrons from FADH2. Cytochrome a3 transfers the electrons to oxygen. a. Four protons are also transferred to oxygen, and two water molecules are the final product of electron transport. b. Cyanide poison kills cells by blocking electron transport and energy production in cells. The three major electron transfer complexes (I, III, and IV) pump H+ ions across the inner membrane. a. Hydrogen ion concentrations build in the inner membrane space. b. The diffusional/electrical gradient that builds produces stored energy called proton-motive force that is ultimately used to produce ATP. Chemiosmosis powers ATP synthesis. a. The inner mitochondrial membrane contains an enzyme called ATP synthase. b. Peter Mitchell first hypothesized that the chemical gradient of the inner membrane space was associated with driving the production of ATP. The ATP synthase enzyme has a complex structure. a. A basal unit is embedded in the inner membrane and is connected to a headpiece that extends into the mitochondrial matrix. b. The basal unit forms a channel through which H+ ions can pass freely. c. The headpiece phosphorylates ADP to ATP, using the proton-motive force of H+ ions moving from the inner membrane space to the mitochondrial matrix. New theories on energy production are emerging. a. Paul Boyer (UCLA) has suggested that movement of hydrogen ions through the basal unit makes the headpiece spin like a top, change shape, and pick up ADP and phosphate then combining them into ATP. b. John Walker (Laboratory of Molecular Biology in Cambridge) verified Boyer’s theory by making an X-ray diffraction 3D model of the ATP synthase enzyme showing the different rotational positions as ATP production occurs. Thirty-two molecules of ATP are produced for each molecule of glucose oxidized to carbon dioxide and water. a. Approximately 2.5 ATP are produced per NADH molecule, and 1.5 ATP are produced per FADH2 that releases electrons to the electron carriers. b. Substrate-level phosphorylation produces a total of 4 ATP during glycolysis and the citric acid cycle. c. Ten NADH and two FADH are eventually used for electron transport and chemiosmosis resulting in the production of 28 ATP. d. Six carbon dioxide molecules are produced per molecule of glucose consumed. Some of the energy associated with NADH produced in the cytoplasm by glycolysis may be lost. a. One shuttle system for cytoplasmic NADH transfers the electrons directly to matrix NAD+ molecules, and no energy is lost. b. One shuttle system for cytoplasmic NADH transfers the electrons to matrix FAD molecules, and the full energy potential of the electrons is lost (~ 30 ATP produced per glucose molecule). c. Different cell types have different NADH shuttles (e.g., heart vs. brain cells). Cellular respiration is only about 30% or so efficient. a. Glucose burned in air results in 686 kcal/mol of energy. b. Glucose oxidized in cells yields 224 kcal/mol of energy. c. The chemical energy not captured is released as heat. Some tissues have uncoupling proteins (UCPs) in the mitochondrial inner membrane that allow hydrogen ions to flow into the matrix without producing ATP. a. Much more energy is lost as heat during such uncoupling of hydrogen gradients. Woelker 2009 b. Brown fat in hibernating animals and in newborn humans as well as some plant cells use these UCPs to produce needed heat. 8.5 Fermentation A. Fermentation is an anaerobic pathway of ATP production. 1. Electrons from NADH are passed to organic acceptor molecules when oxygen is unavailable. 2. Glycolysis can at least continue to help supply ATP by substrate-level phosphorylation. B. There are two major fermentation pathways. 1. Lactate fermentation converts pyruvate to lactate. a. The process often occurs following strenuous activity by muscle cells. b. Lactate can be converted back to pyruvate when oxygen is again available and oxidative phosphorylation can continue. c. Some bacteria ferment glucose to lactate and can be detected in soured milks and yogurts. 2. Alcoholic fermentation occurs in some yeasts. a. In a two step reaction, pyruvate is converted to ethyl alcohol and carbon dioxide. b. Bakers induce yeasts to ferment sugars and produce gases (carbon dioxide) that causes bread to rise, and winemakers induce cultivated yeasts to produce alcohol from grape sugars. C. There are generalized types of organisms based on oxygen needs for metabolism. 1. Strict anaerobes use fermentation as their sole means of ATP production. a. Most require an oxygen free environment for survival. b. The bacterium that causes botulism, for example, thrives in the oxygen free environment of canned foods. 2. Facultative anaerobes can switch from fermentation to oxidative pathways. a. Examples include Escherichia, Lactobacillus, and Saccharomyces. b. Some cells of eukaryotes, such as vertebrate muscle cells, are also facultative. 3. Some prokaryotes and eukaryotes are strict aerobes and can only make ATP in the presence of oxygen. Woelker 2009 Harvesting Chemical Energy: Cellular Respiration 73