* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Increased Expression of Cytoskeletal, Linkage, and Extracellular
Cellular differentiation wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Tissue engineering wikipedia , lookup
Protein moonlighting wikipedia , lookup
Microtubule wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
Proteolysis wikipedia , lookup
Western blot wikipedia , lookup
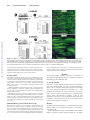
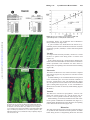
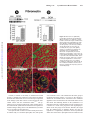
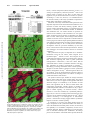
Increased Expression of Cytoskeletal, Linkage, and Extracellular Proteins in Failing Human Myocardium Annette Heling, René Zimmermann, Sawa Kostin, Yoshi Maeno, Stefan Hein, Bruno Devaux, Erwin Bauer, Wolf-Peter Klövekorn, Martin Schlepper, Wolfgang Schaper, Jutta Schaper Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 Abstract—Experimental studies have shown that in hypertrophy and heart failure, accumulation of microtubules occurs that impedes sarcomere motion and contributes to decreased ventricular compliance. We tested the hypothesis that these changes are present in the failing human heart and that an entire complex of structural components, including cytoskeletal, linkage, and extracellular proteins, are involved in causing functional deterioration. In explanted human hearts failing because of dilated cardiomyopathy (ejection fraction ⱕ20%), expression of ␣- and -tubulin, desmin, vinculin, fibronectin, and vimentin was determined by Northern and Western blot analysis and compared with normal myocardium from explants not used for transplantation. The mRNA for ␣- and -tubulin was increased to 2.4-fold (P⬍0.01) and 1.25-fold (NS), respectively; for desmin, 1.2-fold (P⬍0.05); for fibronectin, 5-fold (P⬍0.001); and for vimentin, 1.7-fold (P⬍0.05). Protein levels for ␣-tubulin increased 2.6-fold (P⬍0.02); for -tubulin, 1.2-fold (P⬍0.005); for desmin, 2.1-fold (P⬍0.001); for vinculin, 1.2-fold (P⬍0.005); for fibronectin, 2.9-fold (P⬍0.001); and for vimentin, 1.5-fold (P⬍0.005). Confocal microscopy showed augmentation and disorganization of all proteins studied. In combination with the loss of myofilaments and sarcomeric skeleton previously reported, these changes suggest cardiomyocyte remodeling. Increased fibronectin and elevated interstitial cellularity (vimentin labeling) indicate progressive fibrosis. The present results suggest a causative role of cytoskeletal abnormalities and myofilament loss for intrinsic contractile and diastolic dysfunction in failing hearts. (Circ Res. 2000;86:846-853.) Key Words: heart failure 䡲 cardiomyopathy 䡲 cytoskeleton 䡲 fibrosis 䡲 structure E nd-stage human chronic heart failure is the common final manifestation of a group of different diseases (such as ischemic heart disease, hypertension, valve defects, dilated cardiomyopathy, and others), and it is usually accompanied by myocardial hypertrophy.1 Only a few animal models exist to study this pathophysiologic situation, and these usually are not able to mimic the slowly developing human situation. Therefore, most studies concerning the mechanism of reduced function are carried out in tissue samples from human hearts obtained at the time of cardiac transplantation, either from ischemic hearts or from those with dilated cardiomyopathy (DCM). In this study, we present data obtained in failing human hearts with DCM. In the late stages of this disease, numerous degenerative structural alterations of the cardiomyocytes, as well as areas of focal fibrosis, are scattered throughout the ventricular walls.2– 4 Decompensated hypertrophy occurs when increased cardiac mass fails to normalize wall stress,5 and it has been postulated that in the transition of compensated hypertrophy to a decompensated state, the cardiomyocyte cytoskeleton plays a key role in causing contractile and diastolic dysfunction.6 – 8 Alterations of the cytoskeleton have been described in many excellent studies, by Tsutsui et al6,7 and Tagawa et al,8 –11 in hypertrophied and failing right ventricles of feline6,7,9 –11 and canine8 myocardium. It was shown that the increase in tubulin occurs at the mRNA as well as at the protein level and that an increased viscosity and stiffness of the cardiomyocytes is the consequence of increased amounts and abnormal polymerization of microtubules.11 Evidence was presented that increased microtubular density imposes an intracellular load on the cardiac myocyte that will result in impediment of sarcomere motion and contribute to decreased compliance of the hypertrophied myocardium. In another study, microtubule stabilization and the elevated expression of microtubule-associated protein-4 have been associated with contractile dysfunction.12 It was furthermore shown that accumulation of -tubulin and contractile dysfunction may be related to the mechanical forces imposed on the myocardium during the onset and progression of pressure overload.13 The view that increased microtubular stabilization may be an important factor in causing cellular stiffness and contractile dysfunction in pressure-overload hypertrophy is shared by Wang et al.14 On the other hand, Collins et al,15 investigating cytoskeletal gene expression in a similar experimental model, found the opposite. The authors concluded that neither the level of Received October 12, 1999; accepted February 25, 2000. From the Department of Experimental Cardiology (A.H., S.K., Y.M., M.S., W.S., J.S.), Max Planck Institute, and Department of Cardiac Surgery (R.Z., S.H., E.B., W.-P.K.), Kerckhoff-Clinic, Bad Nauheim, Germany, and Centre Hospitalier (B.D.), Université de Rouen, France. Correspondence to Jutta Schaper, MD, Department of Experimental Cardiology, Max-Planck-Institute, Benekestrasse D-61231, Bad Nauheim, Germany. E-mail [email protected] © 2000 American Heart Association, Inc. Circulation Research is available at http://www.circresaha.org 846 Heling et al TABLE 1. Cytoskeleton in Heart Failure 847 Clinical Characteristics of the Patient Population No. of Patients Sex Age, Years LVEDP, mm Hg EF, % PAP Mean, mm Hg LVEDD, mm DCM (n⫽19) 17 male/2 female 50.8⫾8.3 29.1⫾9.1 16⫾3.9 30.1⫾8.5 69.9⫾5.6 Control (n⫽4) 4 male 48⫾10 8–12 65–70 15–18 35–56 All patients with DCM were treated with digitalis, angiotensin-converting enzyme inhibitors, -blockers, and diuretics at individual dosages. Depending on the individual situation of the patients, treatment with antiarrhythmic drugs was added. EF indicates ejection fraction; PAP, pulmonary arterial pressure; and LVEDD, left ventricular end-diastolic diameter. Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 -tubulin nor its polymerization state affected the contractile performance of the overloaded left ventricle. The role of tubulin accumulation and polymerization was also questioned by Bailey et al,16 who failed to find an effect of colchicine on contraction dynamics in isolated feline myocytes from hypertrophied hearts, and by de Tombe,17 who studied the effects of colchicine and taxol on rat trabecula contractility. In 1991, our group described accumulation and disorganization of microtubules and desmin filaments in human tissue with chronic heart failure.3 In continuation of this work, we tested the hypothesis and present here quantitative protein and mRNA data that show that an entire system of structural proteins, including the cytoskeleton and proteins linking the intracellular milieu to the extracellular matrix such as vinculin and fibronectin, are involved in causing the functional and structural deterioration characteristic of heart failure. Quantification was done by densitometry either by using the gelanalyzing program Quantity One (Protein Imageware System) or by scanning the immunoblots on a STORM 860 imager (Amersham Pharmacia Biotech) using ImageQuant software. All data were expressed as mean⫾SEM. Statistical analysis by unpaired t test was considered significant when P⬍0.05. Standardization To exclude the possibility that the tubulin measured originated from an increased number of fibroblasts in fibrotic tissue, myocardium was first evaluated by light microscopy, and samples showing absence of fibrosis were selected. However, the contribution of an Materials and Methods Nineteen patients with end-stage heart failure (ejection fraction ⱕ20%) due to DCM without any evidence of myocardial infarction undergoing heart transplantation were studied. Hearts from donors with normal left ventricular function not used for transplantation served as controls. The study was approved by the institutional ethical committee. All clinical data are presented in Table 1. Tissue Sampling At the time of transplantation, tissue was removed from the left ventricular free wall excluding papillary muscles. Up to 3 samples were used for either Western or Northern blot and 5 to 8 samples for confocal microscopy. Samples for Western blot were also examined by microscopy. Samples were immediately frozen in liquid nitrogen and stored at – 80°C until further use. Western Blot All antibodies used for Western blot and immunofluorescence are listed in Table 2. SDS-PAGE (separating gels 12%) and immunoblotting were carried out following routine protocols. At least 2 different gels were prepared from each heart. For each lane, 30 g of protein was loaded. Tissue samples from 3 donors served as control. TABLE 2. Antibodies Used for Western Blotting (WB) and Immunofluorescence (IF) Antigen Clone Host Dilution:WB/IF Company Desmin DE-U-10 Mouse 1:100/20 Sigma Vinculin hVIN-1 Mouse 1:100/40 Sigma ␣-Tubulin DM 1A Mouse 1:100/100 Sigma -Tubulin 2-28-33 Mouse 1:100/100 Sigma Fibronectin Polyclonal Rabbit 1:100/100 ICN Vimentin Vg Mouse 1:100/10 Immunotech Pan-actin HHF35 Mouse 1:100 DAKO Figure 1. Illustration of the localization of the proteins investigated. a, Isolated rat myocyte shows network-like longitudinally oriented localization of -tubulin. b, Schematic representation of localization of desmin around the Z-disks of the sarcomeres. c, Localization of vinculin (Vc) as a link between the intracellular milieu and extracellular fibronectin via the integrins of the sarcolemma. Tubulin is present also in fibroblasts. Vimentin present in fibroblasts and endothelial cells is not shown here. 848 Circulation Research April 28, 2000 Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 Figure 2. mRNA for ␣- and -tubulin is significantly increased (A and C) as compared with control (con). Protein levels in failing hearts are elevated for both isoforms in the Western blot (B and D). Blots were carried out for all 19 failing hearts and the controls, but only representative lanes are shown in Figures 2, 3, 5, 6, and 7. E and F, Immunofluorescence for ␣-tubulin (green). E, In normal myocardium, the myocytes are regularly arranged and the tubulin fluorescence is present as a fine network in each cell. F, In failing myocardium, the tubulin fluorescence is more dense and irregular in numerous cells as compared with panel E. increased number of fibroblasts cannot be entirely excluded. Immunoblotting for pan-actin was performed, and only samples showing a similar density of the actin band at 42 kDa were used in this study. The total protein applied to the gel was kept constant. were superimposed for reconstruction in a 3-dimensional mode using a Silicon Graphics Indy workstation and 3-dimensional multichannel image processing software (Bitplane). Northern Blot All proteins studied, with the exception of vimentin, are schematically represented in Figure 1. Vimentin is present in fibroblasts and in endothelial cells. Results The following molecular probes were used for Northern blot analysis: human ␣- and -tubulin and human vimentin (American Type Culture Collection), human desmin (R. Zimmermann, unpublished), rat fibronectin clone pRCabFN1 (K. Boheler, National Heart and Lung Institute, London, UK), and a murine 18S ribosomal RNA cDNA probe (a gift of I. Oberbäumer, Max-Planck-Institut, Martinsried, Germany). Standard methods for Northern blots were used.18 Each gel was done at least in duplicate to control for variability within and between gels. For each well, 15 g of total RNA was loaded. Tissue samples from 3 donors served as control. Quantification of mRNA levels was performed using a PhosphorImager and ImageQuant software (Molecular Dynamics). For normalization, the data of each hybridization signal were divided by the matching 18S signal. All data are presented as mean⫾SEM. Expression was assessed by an unpaired t test. Statistical significance was accepted as P⬍0.05. Both the ␣- and -tubulin isoforms showed an upregulation in the Northern blot, 2.4- and 1.25-fold respectively, and 2.6- and 1.2-fold in Western blot (Figures 2A through 2D). The immunocytochemical localization of ␣- and -tubulin demonstrated an increased density of microtubules in most of the cardiomyocytes as compared with control tissue. Microtubules, normally visible as fine filamentous structures, were disorganized and aggregated, especially in the perinuclear area, ie, microtubular density was increased (Figures 2E and 2F). Immunolabeling and Confocal Microscopy Desmin From each left ventricle, 5 to 8 randomly chosen samples were prepared as previously described.19 Omission of primary antibodies served as negative controls. Preparations were viewed in a confocal laser microscope (Leica) equipped with appropriate filter blocks. The optical confocal sections taken through the depth of tissue samples at 0.5- to 1-m intervals were viewed and photographed individually or Northern blot analysis exhibited a 1.2-fold increase of desmin mRNA (Figure 3A), Western blot showed a 2.1-fold elevation (Figure 3B). In the confocal microscope, a distinct desmin cross-striation pattern is typical of normal myocytes (Figure 3C). In failing ␣- and -Tubulin Heling et al Cytoskeleton in Heart Failure 849 Figure 4. A good correlation exists between LVEDP and cytoskeletal protein content (tubulin and desmin). Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 myocardium, desmin was disorganized and accumulated in many cardiomyocytes (Figure 3D). A good correlation was found between the amount of cytoskeletal proteins (desmin and tubulin) and the left ventricular end-diastolic pressure (LVEDP) in controls and DCM patients (Figure 4). Vinculin As compared with normal myocardium, vinculin was 1.2-fold increased in patients with failing hearts in Western blot (Figure 5A). In the confocal microscope, vinculin showed a distinct localization at the intercalated disks and at the lateral sarcolemma in normal myocardium. This localization was more intense in failing hearts, corresponding to the results from Western blotting (Figure 5B). Fibronectin The mRNA for fibronectin was 5-fold increased as compared with controls (Figure 6A); the protein was increased 2.9-fold (Figure 6B). In immunolabeling, it was evident that fibronectin was present in the extracellular space and in the basement membrane of myocytes, smooth muscle cells, and endothelial cells (Figure 6C). The expression was more intense in the widened interstitial space of failing hearts (Figure 6D). A close colocalization of fibronectin and vinculin was shown by double labeling (Figures 6E and 6F) Vimentin Figure 3. Desmin is significantly increased on the mRNA and protein levels as shown by Northern (A) and Western (B) blot analysis (con indicates control). C and D, Confocal microscopy for desmin. C, Normal human myocardium shows a regular cross-striation at the Z-level and distinct intercalated disk labeling. D, In failing myocardium, the cross-striation pattern is disturbed and desmin is accumulated in many irregularly shaped cells. Nuclei are red. The mRNA for vimentin was upregulated 1.7-fold as compared with controls (Figure 7A), and the protein was increased 1.5-fold in patients with heart failure (Figure 7B). Immunostaining of normal myocardium showed that vimentin was present in fibroblasts and endothelial cells (Figure 7C). In failing hearts, fibroblasts were more numerous, corresponding to the values from Western blot (Figure 7D). Discussion This study demonstrates for the first time significant changes of cytoskeletal and membrane-associated proteins in human hearts failing because of longstanding DCM. We show that 850 Circulation Research April 28, 2000 Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 Figure 5. A, Immunoblotting shows that vinculin is significantly increased in failing myocardium (con indicates control). B and C, Vinculin localization using confocal microscopy. B, In normal myocardium, labeling at the intercalated disk, at the costameres, and in the T-tubular system is evident. C, In failing myocardium, vinculin fluorescence is more abundant and disorganized. Red in panel C indicates lipofuscin granules. tubulin and desmin are significantly increased, not only at the protein level but also at the mRNA level. The same is true for vinculin, fibronectin, and vimentin. In the confocal microscope, a distinct disorganization and accumulation of the microtubules and desmin filaments were evident, which supports the quantitative protein data. A good correlation was found between protein content and LVEDP in controls and patients with DCM. It is hypothesized, therefore, that not only the loss of myofilaments as described previously2,3 but also the increase in cytoskeletal and membrane-associated proteins may play a major role in the pathogenesis of contractile and diastolic dysfunction in failing hearts.4 The role of the cytoskeleton as a stabilizing factor of cellular structure and functional integrity is well established.20 Tubulin is organized around the nucleus and in the longitudinal direction of the cell, and it contributes to the stability of the contractile apparatus in relation to the nucleus, mitochondria, and cellular membrane.21 Tubulin turnover is continuous and high and is an energy-consuming process. Therefore, it is especially interesting that a ubiquitous accumulation of tubulin takes place in failing hearts. It cannot be excluded at the present time that tubulin synthesis plays a significant role in the transition from hypertrophy to the decompensated state in the human heart, as shown in rats with developing hypertrophy due to aortic banding.22 The concept of microtubular involvement in contractile and diastolic dysfunction in failing hearts, previously formulated by Tsutsui et al6,7 and Tagawa et al11 in hearts with decompensated hypertrophy, is supported and extended to the involvement of other proteins by the data in human hearts presented here. Cellular hypertrophy as present in experimental animals was also found in our patients.23 One significant difference between both models, however, is the fact that in the animal experiments the contractile machinery was intact, indicating that the more chronic situation in the human heart represents a unique pathophysiological entity. The present data suggest that similarities develop in the final structural as well as functional deterioration in failing hearts independently of the cause of failure. It may be concluded that changes, not only of tubulin, as reported by Tsutsui et al6,7 and Tagawa et al,11 but also of desmin and other linkage proteins, in addition to the loss of myofilaments and the occurrence of fibrosis, are the structural correlate of the reduction of ejection fraction and the increase in LVEDP as observed in our patients. Desmin connects the sarcomeres in the transverse direction, thereby inhibiting slippage during contraction. Desmin accumulation and disorganization were especially evident in cells lacking contractile material. Given that desmin is localized around Z-lines and that it connects neighboring sarcomeres, this disorganization can be explained by the absence of a structural element with which it can be associated. Using desmin knockout mice, Capetanaki et al24 showed that desmin is essential for the maintenance of the functional integrity of myofibrils, whole myofibers, and the general cellular integrity, which was confirmed in mice with desmin null mutations in which degeneration of cardiac muscle was observed.25 The findings of the present study are in agreement with those reported in congenital myopathies and hereditary cardiac diseases.26–28 Therefore, the desmin augmentation found in human myocardium was interpreted as a compensatory mechanism to ensure cellular integrity and sarcomere stability. Heling et al Cytoskeleton in Heart Failure 851 Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 Figure 6. Fibronectin is significantly increased by Northern (A) and Western (B) blot analysis (con indicates control). C and D, Confocal images of fibronectin (green) and actin (red) labeling. E and F, Colocalization of fibronectin (red) and vinculin (green). C, Normal myocardium shows fibronectin as a fine line surrounding the myocytes and capillary vessels. D, In failing myocardium, the interstitial space is enlarged and fibronectin is significantly increased, leading to encapsulation of myocytes. E, In this slightly tangential section, vinculin is present at the intercalated disk, at the lateral sarcolemma, and in the T system. F, At high magnification of the boxed area from panel E, the close spatial localization of fibronectin and vinculin at the T-tubular membrane and the sarcolemma is evident (arrows). Myocytes are unstained (black). Vinculin, a member of the family of membrane-associated proteins, is situated at the costameres of the lateral sarcolemma and at the intercalated disk19 and is one of the major components of the linkage system that connects via the integrins the intracellular milieu with the extracellular matrix.29 –31 The 1integrins act as receptors for fibronectin. Their dynamic interaction with both the cytoskeleton and fibronectin is important for structural changes during muscle cell differentiation (see Reference 32 for other relevant references). We report here that vinculin was increased at the protein level. In another study, we found that dystrophin, talin, and spectrin were increased as well.21 This indicates that the entire group of membrane-associated proteins is involved in the attempt to stabilize the sarcolemma and its connections to the contractile apparatus on the one hand and to the extracellular matrix on the other hand. The stabilizing function of the cytoskeleton is of importance when the contractile material of the cardiomyocytes is reduced as a result of the progression from hypertrophy to failure. In the early stages of the disease, therefore, this might help the cardiomyocytes to maintain function and may be regarded as a compensatory mechanism. Recent experimental studies have shown that a dystrophin missense mutation in the 852 Circulation Research April 28, 2000 Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 Figure 7. Vimentin is significantly increased on the mRNA (A) and protein (B) levels (con indicates control). C and D, Confocal images of immunofluorescence for vimentin (red), with myocytes counterstained with phalloidin (green). C, Normal myocardium with few fibroblasts and capillaries. D, In failing myocardium, the myocytes are separated by fibrotic material containing many fibroblasts. Only few capillaries are visible. Red in myocytes indicates lipofuscin granules. mouse,33 muscle LIM protein (MLP) deficiency in mice,34 or cleavage of dystrophin by enteroviral proteases35 result in the clinical picture of DCM. On the basis of these data, the hypothesis was put forward that DCM is a disease of cytoskeletal damage.36,37 This view, however, is in contradistinction to the increased density of the major cytoskeletal components in human hearts with DCM as reported here. Fibronectin is the major extracellular matrix protein and is an important component of the cellular basement membrane.38 It is increased in hearts with DCM, resulting, together with an increase of other extracellular proteins, in a significant degree of fibrosis.4,39 Vimentin, the intermediate filament of fibroblasts and endothelial cells, was studied because it represents an indicator of the cellularity of the interstitium.40 It was significantly elevated because the number of fibroblasts was higher than in control. Taken together, these findings indicate that the synthesis of connective tissue components is continuous and will result in progressive fibrosis despite pretransplantation treatment of all patients with angiotensin-converting enzyme inhibitors. In an experimental study, we have recently shown that fibrosis development cannot be prevented indefinitely by this treatment,41 which would confirm the data presented here. Fibrosis will contribute to an increased stiffness and loss of ventricular compliance.42 The cytoskeleton also plays an important role in mechanotransduction across the cell surface, where integrins act as mechanoreceptors.43 Transfer of force from integrins to the cytoskeleton is thought to represent a proximal step in intracellular mechanical signaling that leads to global cytoskeletal rearrangements.43 Furthermore, it has been shown that the extracellular matrix controls cytoskeletal mechanics and structure, particularly by binding of fibronectin to integrins.44,45 Interestingly, increasing extracellular matrix contacts by binding through an elevated number of integrins increases cytoskeletal stiffness and apparent viscosity.44 Transferring these data to our own findings, it may be assumed that the increased content of fibronectin found in failing hearts is the inducer of changes of cytoskeletal structure and function. The question remains open whether the changes described here are effects rather than causes of heart failure. The present data, however, are more in support of the hypothesis that these structural changes are contributory to the transition to heart failure. This hypothesis will be tested in human hearts with compensatory hypertrophy. Another question is whether the cytoskeletal proteins either have a structural role only or participate in cellular signaling. As discussed recently, “chronic increases in wall stress mediated by cytoskeletal pathways” (page 558) as is the case in failing hearts with DCM, may play a decisive role in cellular hypertrophy and cell survival.46 Recent evidence concerning ␣- or ␦-sarcoglycan47 or LIM domain protein34 – deficient mice strongly suggests that the cytoskeleton might be involved in cellular signaling. In conclusion, in myocytes from failing human hearts, the major components of the cytoskeleton tubulin and desmin, as well as the membrane-associated protein vinculin, are increased, most probably as a mechanism compensatory for the loss of contractile filaments, thereby contributing to an increased stiffness of individual cardiomyocytes. Furthermore, the linkage protein vinculin and the extracellular matrix protein fibronectin Heling et al are augmented, which will, by interaction with the integrins, further increase cellular stiffness. Fibronectin and vimentin indicate a progressive development of fibrosis. The present data support experimental reports showing the important role of microtubule accumulation in failing hearts. They furthermore show that changes of a whole complex of proteins, in addition to the loss of contractile filaments and proteins of the sarcomeric skeleton described previously,3 represent the structural basis for the reduction of contractile function and compliance in failing human hearts.21 Acknowledgment We thank Gunther Schuster for computer assistance. References Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 1. Braunwald E. Pathophysiology of heart failure. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pa: WB Saunders Co Inc; 1980:453– 483. 2. Hein S, Scholz D, Fujitani N, Rennollet H, Brand T, Friedl A, Schaper J. Altered expression of titin and contractile proteins in failing human myocardium. J Mol Cell Cardiol. 1994;26:1291–1306. 3. Schaper J, Froede R, Hein S, Buck A, Hashizume H, Speiser B, Friedl A, Bleese N. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83:504–514. 4. Schaper J, Speiser B, Brand T. The cytoskeleton and extracellular matrix in human hearts with dilated cardiomyopathy. In: Figulla HR, Kandolf R, McManus B, eds. Idiopathic Dilated Cardiomyopathy. Berlin, Germany: Springer-Verlag; 1993:75–80. 5. Grossman W, Jones D, McLaurin MP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. 6. Tsutsui H, Ishihara K, Cooper G. Cytoskeletal role for contractile dysfunction of hypertrophied myocardium. Science. 1993;260:682–687. 7. Tsutsui H, Tagawa H, Kent RL, McCollam PL, Ishihara K, Nagatsu M, Cooper G. Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation. 1994;90:533–555. 8. Tagawa H, Koide M, Sato H, Zile MR, Carabello BA, Cooper G. Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ Res. 1998;82:751–761. 9. Tagawa H, Koide M, Sato I, Cooper G. Cytoskeletal role in the contractile dysfunction of cardiocytes from hypertrophied and failing right ventricular myocardium. Proc Assoc Am Physicians. 1996;108:218–229. 10. Tagawa H, Rozich JD, Tsutsui H, Narishige T, Kuppuswamy D, Sato H, McDermott PJ, Koide M, Cooper G. Basis for increased microtubules in pressure-hypertrophied cardiocytes. Circulation. 1996;93:1230–1243. 11. Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res. 1997;80:281–289. 12. Sato II, Nagai T, Kuppuswamy D, Narishige T, Koide M, Menick DR, Cooper G. Microtubule stabilization in pressure overload cardiac hypertrophy. J Cell Biol. 1997;139:963–973. 13. Watson PA, Hannan R, Carl LL, Giger KE. Contractile activity and passive strength regulate tubulin mRNA and protein content in cardiac myocytes. Am J Physiol. 1996;271(2 pt 1):C684–C689. 14. Wang X, Li F, Campbell SE, Gerdes M. Chronic pressure overload hypertrophy and failure in guinea pigs, II: cytoskeletal remodeling. J Mol Cell Cardiol. 1999;31:319–331. 15. Collins JF, Pawloski-Dahm C, Davies MG, Ball N, Dorn GW, Walsh RA. The role of the cytoskeleton in left ventricular pressure overload hypertrophy and failure. J Mol Cell Cardiol. 1996;28:1435–1443. 16. Bailey BA, Dipla K, Li S, Houser SR. Cellular basis of contractile derangements of hypertrophied ventricular myocytes. J Mol Cell Cardiol. 1997;29:1823–1835. 17. de Tombe PP. Altered contractile function in heart failure. Cardiovasc Res. 1998;37:367–380. 18. Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987; 162:156–159. 19. Kostin S, Scholz D, Shimada T, Maeno Y, Mollnau H, Hein S, Schaper J. The internal and external protein scaffold of the T-tubular system in cardiomyocytes. Cell Tissue Res. 1998;294:449–460. Cytoskeleton in Heart Failure 853 20. Stromer MH. The cytoskeleton in skeletal, cardiac and smooth muscle cells. Histol Histopathol. 1998;13:283–291. 21. Kostin S, Heling A, Hein S, Scholz D, Klövekorn W-P, Schaper J. The protein composition of the normal and diseased cardiac myocyte. Heart Fail Rev. 1998;2:245–260. 22. Watkins SC, Samuel JL, Marotte F, Bertier-Savalle B, Rappaport L. Microtubules and desmin filaments during onset of heart hypertrophy in the rat: a double immunoelectron microscope study. Circ Res. 1987;60:327–336. 23. Scholz D, Diener W, Schaper J. Altered nucleus/cytoplasm relationship and degenerative structural changes in human dilated cardiomyopathy. Cardioscience. 1994;5:127–138. 24. Capetanaki A, Milner DJ, Weitzer G. Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct Funct. 1997;22: 103–116. 25. Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. 26. Thornell LE, Johanson B, Erikson A, Lehto VP, Virtanen I. Intermediate filament and associated proteins in the human heart: an immunofluorescence study of normal and pathological hearts. Eur Heart J. 1984;5(suppl F):231–241. 27. Thornell LE, Price MG. The cytoskeleton in muscle cells in relation to function. Biochem Soc Transact. 1991;19:1116–1120. 28. Thornell LE, Carlsson L, Li Z, Mericskay M, Paulin D. Null mutation in the desmin gene gives rise to a cardiomyopathy. J Mol Cell Cardiol. 1997;29: 2107–2124. 29. Geiger B. Vinculin, an intracellular protein localized at specialized sites where microfilaments bundles terminate at cell membranes. Proc Natl Acad Sci U S A. 1980;77:4127–4131. 30. Craig SW, Pardo JV. Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motil. 1983;3:449–462. 31. Pardo JV, Siliciano J, Craig SW. Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol. 1983;97:1081–1088. 32. Enomoto MI, Boettiger D, Menko AS. ␣5-Integrin is a critical component of adhesion plaques in myogenesis. Dev Biol. 1993;155:180–197. 33. Ortiz-Lopez R, Hua L, Su J, Goytia V, Towbin JA. Evidence for a dystrophin missense mutation as a cause of X-linked dilated cardiomyopathy. Circulation. 1997;95:2434–2440. 34. Arber S, Hunter JJ, Ross J Jr, Hongo M, Sansig G, Borg J, Perriard J-C, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1987; 88:393–403. 35. Badorff C, Lee GH, Lamphear BL, Martone ME, Campbell KP, Rhoads RE, Knowlton KU. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5: 320–326. 36. Towbin JA. The role of cytoskeletal proteins in cardiomyopathies. Curr Opin Cell Biol. 1998;10:131–139. 37. Towbin JA, Bowles KR, Bowles NE. Etiologies of cardiomyopathy and heart failure. Nat Med. 1999;5:266–267. 38. Hynes RO. Fibronectins. Berlin, Germany: Springer; 1989. 39. Schaper J, Hein S, Scholz D, Mollnau H. Multifaceted morphological alterations are present in the failing human heart. J Mol Cell Cardiol. 1995;27: 857–861. 40. Traub P. Intermediate Filaments: A Review. Berlin, Germany: SpringerVerlag; 1985. 41. Zimmermann R, Kastens J, Linz W, Wiemer G, Schölkens BA, Schaper J. Effect of long-term ACE inhibition on myocardial tissue in hypertensive stroke-prone rats. J Mol Cell Cardiol. 1999;31:1447–1456. 42. Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27:341–348. 43. Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. 44. Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. 45. Choquet D, Felsenfeld DP, Sheetz MP. Extracellular-matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. 46. Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. 47. Coral-Vazquez R, Cohn RD, Moore SA, Hill JA, Weiss RM, Davisson RL, Straub V, Barresi R, Bansal D, Hrstka RF, Williamson R, Campbell K. Disruption of the sarcoglycan-sarcospan complex in vascular smooth muscle: a novel mechanism for cardiomyopathy and muscular dystrophy. Cell. 1999; 98:465–474. Downloaded from http://circres.ahajournals.org/ by guest on June 16, 2017 Increased Expression of Cytoskeletal, Linkage, and Extracellular Proteins in Failing Human Myocardium Annette Heling, René Zimmermann, Sawa Kostin, Yoshi Maeno, Stefan Hein, Bruno Devaux, Erwin Bauer, Wolf-Peter Klövekorn, Martin Schlepper, Wolfgang Schaper and Jutta Schaper Circ Res. 2000;86:846-853 doi: 10.1161/01.RES.86.8.846 Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2000 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7330. Online ISSN: 1524-4571 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/content/86/8/846 Data Supplement (unedited) at: http://circres.ahajournals.org/content/suppl/2000/07/19/86.8.846.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation Research is online at: http://circres.ahajournals.org//subscriptions/