* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1.3 Diffusion, Osmosis, and the Cell Membrane • Diffusion is the
Survey
Document related concepts
Transcript
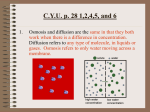
1.3 Diffusion, Osmosis, and the Cell Membrane • Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. • Concentration is the amount of substance in a given space. • The smell of fresh baked bread “spreading” throughout the room is an example of diffusion. The diffusion of ink in water. See pages 40 - 41 (c) McGraw Hill Ryerson 2007 Diffusion and the Cell Membrane • The cell membrane is a selectively permeable membrane. w This means that it has many small openings that let some substances pass through it but not others. • One way that substances can move through the cell membrane is by diffusion. • When the concentration on both sides of the membrane is the same, it is called equilibrium. See page 42 (c) McGraw Hill Ryerson 2007 Osmosis • Osmosis is the diffusion of water through a selectively permeable membrane. • Osmosis occurs when water particles move from a higher concentration to a lower concentration. See page 43 (c) McGraw Hill Ryerson 2007 Osmosis and the Cell • Cells contain water and need this water to survive. • Osmosis is how the cell gains and loses its needed water. Explain how placing this wilted flower in water will cause the flower to “straighten up”. See pages 43 - 44 (c) McGraw Hill Ryerson 2007 Examples of Osmosis Example 1: Equal movement of water in and out of cells See page 45 (c) McGraw Hill Ryerson 2007 Examples of Osmosis Example 2: More water moving into cells than is moving out See page 45 (c) McGraw Hill Ryerson 2007 Examples of Osmosis Example 3: More water moving out of cells than is moving in See page 45 (c) McGraw Hill Ryerson 2007