* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A fatty acid in the MCT ketogenic diet for epilepsy treatment blocks
Survey
Document related concepts
Toxicodynamics wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Psychopharmacology wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
NMDA receptor wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Neuropharmacology wikipedia , lookup
Transcript
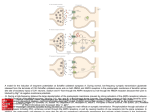
306 | BRAIN 2016: 139; 304–316 Hansen N, Grünewald B, Weishaupt A, Colaço MN, Toyka KV, Sommer C, et al. Human Stiff person syndrome IgGcontaining high-titer anti-GAD65 autoantibodies induce motor dysfunction in rats. Exp Neurol 2013; 239: 202–9. Irani SR, Gelfand JM, Al-Diwani A, Vincent A. Cell-surface central nervous system autoantibodies: Clinical relevance and emerging paradigms. Ann Neurol 2014; 76: 168–84. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010; 9: 67–76. Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann NY Acad Sci 2015; 1338: 94–114. Scientific Commentaries Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol 2005; 58: 96–107. Sommer C, Weishaupt A, Brinkhoff J, Biko L, Wessig C, Gold R, et al. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet 2005; 365: 1406–11. Tanaka K, Tanaka M, Igarashi S, Onodera O, Miyatake T, Tsuji S. Trial to establish an animal model of paraneoplastic cerebellar degeneration with anti-Yo antibody. 2. Passive transfer of murine mononuclear cells activated with recombinant Yo protein to paraneoplastic cerebellar degeneration lymphocytes in severe combined immunodeficiency mice. Clin Neurol Neurosurg 1995; 97: 101–5. Varley J, Vincent A, Irani SR. Clinical and experimental studies of potentially pathogenic brain-directed autoantibodies: current knowledge and future directions. J Neurol 2015; 262: 1081–95. Werner C, Pauli M, Doose S, Weishaupt A, Haselmann H, Grunewald B, et al. Human autoantibodies to amphiphysin induce defective presynaptic vesicle dynamics and composition. Brain 2016; 139: 365–79. Wessig C, Klein R, Schneider MF, Toyka KV, Naumann M, Sommer C. Neuropathology and binding studies in anti-amphiphysin-associated stiff-person syndrome. Neurology 2003; 61: 195–8. A fatty acid in the MCT ketogenic diet for epilepsy treatment blocks AMPA receptors This scientific commentary refers to ‘Seizure control by decanoic acid through direct AMPA receptor inhibition’, by Chang et al. (doi:10.1093/ brain/awv325). Dietary therapies can provide control of seizures in patients with drugrefractory epilepsy. There are several types of dietary therapies, all of which are high in fat, restrict carbohydrates to some extent, and are associated with ketosis. In the classical ketogenic diet introduced into clinical practice in the 1920s, the fat component is provided by long-chain triglycerides (LCTs). Recognizing that medium chain triglycerides (MCTs) are more ketogenic than LCTs, Huttenlocher et al. (1971) created the MCT oil diet, which permits greater amounts of carbohydrate and protein, and therefore allows a more flexible meal plan. The efficacy of the MCT oil ketogenic diet is equivalent to that of the classic LCT ketogenic diet, and the tolerability is also comparable (Neal et al., 2009). MCTs, which are abundant in coconut and palm kernel oil, have a glycerol backbone and three fatty acid esters with 6 to 12 carbons arranged in a straight chain. The major fatty acids in MCT oil are n-octanoic acid (C8; caprylic acid; 50–80%) and n-decanoic acid (C10; capric acid; 20–50%). Following ingestion, MCTs are rapidly absorbed into the portal circulation to the liver, in contrast to LCTs which are absorbed by chylomicrons in the lymph. It was previously believed that medium chain fatty acids derived from MCTs are immediately oxidized in the liver to form ketone bodies, but it is now known that appreciable amounts appear in the circulation of patients receiving the MCT diet (Sills et al., 1986a). For example, children on the MCT diet had plasma concentrations of decanoic acid in the range of 0.1–0.2 mM. Decanoic acid readily crosses the blood–brain barrier, probably by a combination of diffusion and saturable carrier-mediated transport via a medium-chain fatty acid transporter that also mediates brain uptake of the antiseizure drug valproic acid, itself a medium chain fatty acid (Adkison and Shen, 1996). In this issue of Brain, Chang et al. show for the first time that decanoic acid is an antagonist of AMPA receptors, providing a possible basis for the antiseizure activity of the MCT diet (Chang et al., 2016). A number of theories have been advanced to explain the antiseizure activity of high fat ketogenic diets but none has as yet received broad acceptance (Hartman et al., 2007). Early on, it was proposed that ketone bodies are responsible for seizure protection and indeed all of the ketone bodies (acetone, b-hydroxybutyrate and acetoacetate) have been reported to have antiseizure effects in one or another animal model. However, as confirmed by Chang et al. the ketones are not generally active in in vitro seizure models. Moreover, the activity profile of ketogenic diets in animal models does not correspond with that of the ketones and the degree of ketosis does not correlate with the extent of seizure protection. Other investigators have concluded that the diets enhance GABA synthesis, correcting deficits in inhibitory neurotransmission. More recent studies have implicated increased production of brain bioenergetic substrates such as ATP, creatine and phosphocreatine and increased mitochondrial energy metabolism, which is postulated to Scientific Commentaries BRAIN 2016: 139; 304–316 | 307 NH2 Aminoterminal domain Competitive antagonists D1 Ligand binding domain Linker Glutamate D2 S2-M4 S1-M1 Extracellular side M1 M3 Transmembrane domain Noncompetitive antagonists N M4 N O N M2 Perampanel Cytoplasmic side Decanoic acid O O– HOOC Figure 1 AMPA receptor protein structure illustrating putative locations of binding sites for decanoic acid and non-fatty acid non-competitive antagonists such as perampanel. The AMPA receptor consists of four subunits, each of which is rendered in the structure on the left in a different colour; one of the subunits is shown as a ribbon diagram. Small molecule binding sites are highlighted in the subunit rendered in black lines; similar binding sites exist in the other identical subunits but are not highlighted. Non-competitive antagonists are believed to bind to the S1-M1 linker (residues FLDPLAYE) and the S2-M4 linker; the S1-M1 residues are highlighted as light grey spheres. By molecular modelling, Chang et al. determined that a likely site for binding of decanoic acid is to the M3 segment in the transmembrane domain; key residues near the cytoplasmic side (PRSL_GR) are highlighted as dark grey spheres. Additional likely residues for binding of decanoic acid to the cytoplasmic sides of the M1 (LFLVS) and M2 (NS_WF) segments are not highlighted. Binding of decanoic acid at these sites could block the channel pore, which is formed by the transmembrane domains of all four subunits. Evidence in favour of a pore-blocking mechanism is the demonstration of increased block at depolarized membrane potentials, since the more positive electrostatic field at the cytoplasmic side of the membrane at depolarized potentials would tend to more strongly attract into the pore the negatively charged fatty acid approaching the channel from the extracellular solution. The dashed arrow indicates the axis of symmetry of the transmembrane domain and the path for ion flow. The structure is 3KG2 (homomeric rat GluA2 in closed antagonist bound state) from the RCSB Protein Data Bank as was used by Chang et al. The illustration on the right schematically represents the various channel domains and the locations of the small molecule binding sites in a single subunit. stabilize neuronal ion gradients, thereby resisting depolarizing influences and consequent seizure generation. In addition, the increased ATP has been proposed to enhance the production of the inhibitory mediator adenosine, which is well recognized to have antiseizure properties. It has also been noted that the diets enhance mobilization of polyunsaturated fatty acids, which may protect against seizures through effects on ion channels. In sum, many of the hypothesized mechanisms of the dietary therapies implicate effects on intermediary metabolism. This is in contrast to the mechanisms of antiseizure drugs, which—with a few exceptions—are believed to act through direct effects on ion channels, leading to reduced neuronal excitation, or on components of the GABA system, leading to enhanced GABA-mediated inhibition. Fast (millisecond timescale) synaptic excitation in the brain is mediated by ionotropic glutamate receptors (a family of ligand-gated ion channels), which are membrane-spanning protein complexes localized at synapses, 308 | BRAIN 2016: 139; 304–316 usually on dendritic spines. Neurotransmitter glutamate released from presynaptic excitatory terminals diffuses across the synaptic cleft to activate ionotropic glutamate receptors in the postsynaptic membrane, leading to entry of cations (Na + and in some cases Ca2 + ), which generates the depolarizing excitatory postsynaptic potential (EPSP). AMPA receptors, which are assembled as heteromeric or homomeric tetramers of the protein subunits GluA1, GluA2, GluA3 and GluA4 (each consisting of 900 amino acids), are responsible for the bulk of the EPSP and there is a variable contribution of NMDA receptors. AMPA receptors are also key mediators of pathological electrochemical events in focal epilepsies, including the paroxysmal depolarization shift (the basis of the EEG spike) and the electrographic seizure discharge. In addition to mediating synchronous discharges in epileptic foci, AMPA receptors are critically important in the spread of seizure activity locally and to distant brain regions. Since the early 1980s, it has been known that pharmacological inhibition of ionotropic glutamate receptors can protect against seizures in experimental models. Early efforts to develop antiseizure agents that inhibit glutamate excitation focused on NMDA receptor antagonists, but disappointing clinical results dampened enthusiasm. There has recently been a resurgence of interest in ionotropic glutamate receptor antagonists with the demonstration in clinical trials that perampanel, a potent selective orally-active non-competitive AMPA receptor antagonist, is effective in the treatment of focal seizures and primary and secondarily generalized tonic-clonic seizures. Medium chain fatty acids, including decanoic acid, have long been known to have acute antiseizure actions in animal models. Valproic acid is a branched medium chain fatty acid that was demonstrated to be effective in clinical trials in the 1960s. The mechanism of action of valproic acid is obscure. Chang et al. now confirm the antiseizure activity of decanoic Scientific Commentaries acid (1 mM) in in vitro rat hippocampal slice models, including a model generated by exposure to the GABAA receptor antagonist pentylenetetrazol (PTZ) and another model in which extracellular Mg2 + is reduced, leading to unblock of NMDA receptors. At concentrations within the range of those present in the blood of patients maintained on the MCT diet (0.3 mM), Chang et al. further demonstrate that decanoic acid selectively blocks excitatory synaptic currents without affecting inhibitory synaptic currents. These results are then followed by the key observation of their study, which is that decanoic acid causes a selective non-competitive inhibition of recombinant AMPA receptors expressed in Xenopus oocytes, but with variable potency depending upon the subunit combination. The effect of decanoic acid was selective in that neither valproic acid nor octanoic acid significantly affected AMPA receptor currents at a concentration of 1 mM. The authors argue that perampanellike effects of decanoic acid are responsible for the antiseizure actions of the MCT diet and that it may not be necessary to invoke any other mechanism. How reasonable is this proposal? If the antiseizure activity of the MCT diet is entirely due to block of AMPA receptors, then the diet could be replaced by perampanel or another AMPA receptor antagonist. There are no headto-head comparisons of perampanel with other antiseizure agents or with the MCT diet. Nevertheless, it is believed by many epileptologists that ketogenic diets, including the MCT diet, are often effective in patients who do not respond to antiseizure drugs (Sills et al., 1986b). These physicians would be surprised if the diet was no more efficacious than an antiseizure drug. The present study suggests that a comparative trial between the MCT diet and an AMPA receptor antagonist would be worthwhile. If it were possible to replace the diet with an AMPA receptor antagonist, this would enormously simplify therapy, avoid the poor palatability and gastrointestinal side effects of the diet, and therefore make treatment available to a broader group of patients. A randomized trial comparing the LCT and MCT ketogenic diets in children with drug-refractory epilepsy failed to find any difference in the number of children who responded to the diet with a reduction in seizures or who achieved seizure freedom, as occurred in a small number of subjects (Neal et al., 2009). This observation is difficult to reconcile with the idea that the MCT diet has a unique mechanism of action. It seems more plausible that the LCT and MCT diets act through a similar set of mechanisms, perhaps with the addition of AMPA receptor blockade by decanoic acid in the case of the MCT diet. There are other observations in the literature that raise further questions. In animal models, decanoic acid does elevate the seizure threshold in some situations but the effect is modest (minimal effective dose is 1723 mg/kg, orally) and there is no effect on PTZ-induced seizures (Wlaź et al., 2015). In contrast, perampanel has potent, broad-spectrum antiseizure activity in diverse models including the PTZ model (ED50, 0.94 mg/kg, orally). Octanoic acid, a quantitatively greater constituent of MCT oil than decanoic acid, did not affect epileptiform discharges in vitro at a concentration of 1 mM (Chang et al., 2013) and also failed to affect AMPA receptor currents in Xenopus oocytes (Chang et al., 2016). However, octanoic acid has long been known to have sedative and antiseizure activities (Liu and Pollack, 1994) and a recent study indicates that it is at least as potent as decanoic acid (Wlaź et al., 2012). Surprisingly, octanoic acid does raise the PTZ threshold (Wlaź et al., 2012). Understanding the antiseizure action of other medium chain fatty acids including valproic acid represents a major scientific challenge. In sum, Chang et al. for the first time invoke AMPA receptors as a potential molecular target in a dietary therapy for epilepsy. AMPA receptors are a clinically validated antiseizure drug target, thus it is plausible that an action on AMPA receptors could contribute to the activity of the MCT diet in epilepsy therapy. Whether this is Scientific Commentaries BRAIN 2016: 139; 304–316 | 309 Glossary AMPA receptor: Ligand-gated non-selective cation channel that allows the flow of Na + , K + and in some cases Ca2 + in response to binding of glutamate; ligands, such as glutamate, that gate (open) the channel are referred to as agonists. Channel block: Functional inhibition of an ion channel by a blocking drug that binds inside the ion conduction pore preventing flow of ions through the channel. Heteromeric receptor/homomeric receptor: Heteromeric applies when at least one subunit is non-identical to the others; homomeric applies when all subunits are identical. Ketone body: Three molecules (acetone, b-hydroxybutyrate and acetoacetate) produced in the liver during the metabolism of fats that serve as an alternative source of energy to glucose. MCT diet: A dietary treatment for epilepsy more commonly used in Europe than in the United States that is high in fats containing predominantly medium chain triglycerides; causes ketosis, a state where there are elevated concentrations of ketone bodies in the blood. Non-competitive antagonist: In the case of ionotropic glutamate receptors, an inhibitor whose blocking action is not reduced progressively as the agonist (glutamate) concentration is increased as occurs for competitive antagonists. Non-competitive antagonists allosterically inhibit the channel by binding to a site other than the ligand binding domain. Triglyceride: The storage form of fatty acids in the body; specifically, an ester derived from glycerol and three fatty acids, such as decanoic acid (capric acid) triglyceride, which is composed of glycerol and three decanoic acid molecules covalently linked. the only mechanism of the MCT diet requires further investigation. Michael A. Rogawski Department of Neurology, School of Medicine, University of California, Davis Sacramento, California, USA E-mail: [email protected] doi:10.1093/brain/awv369 References Adkison KD, Shen DD. Uptake of valproic acid into rat brain is mediated by a medium-chain fatty acid transporter. J Pharmacol Exp Ther 1996; 276: 1189–200. Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016; 139: 431–43. Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013; 69: 105–14. Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol 2007; 36: 281–92. Huttenlocher PR, Wilbourn AJ, Signore JM. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971; 21: 1097–103. Liu M-J, Pollack GM. Pharmacokinetics and pharmacodynamics of valproate analogues in rats. IV. Anticonvulsant action and neurotoxicity of octanoic acid, cyclohexanecarboxylic acid, and 1-methyl-1-cyclohexanecarboxylic acid. Epilepsia 1994; 35: 234–43. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. A randomized trial of classical and medium- chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009; 50: 1109–17. Sills MA, Forsythe WI, Haidukewych D. Role of octanoic and decanoic acids in the control of seizures. Arch Dis Child 1986a; 61: 1173–7. Sills MA, Forsythe WI, Haidukewych D, MacDonald A, Robinson M. The medium chain triglyceride diet and intractable epilepsy. Arch Dis Child 1986b; 61: 1168–72. Wlaź P, Socala K, Nieoczym D, Luszczki JJ, Zarnowska I, Zarnowski T, et al. Anticonvulsant profile of caprylic acid, a main constituent of the medium-chain triglyceride (MCT) ketogenic diet, in mice. Neuropharmacology 2012; 62: 1882–9. _ arnowski Wlaź P, Socala K, Nieoczym D, Z _ T, Zarnowska I, Czuczwar SJ, et al. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog Neuropsychopharmacol Biol Psychiatry 2015; 57: 110–16. When is temporal lobe epilepsy not temporal lobe epilepsy? This scientific commentary refers to ‘Temporal plus epilepsy is a major determinant of temporal lobe surgery failures’, by Barba et al. (doi:10.1093/ brain/awv372). Stereotactic electroencephalography (SEEG) originated in France in the 1950s. Bancaud and Talairach, at St. Anne’s Hospital in Paris, introduced this approach to define the 3D extent of dysfunctional brain tissue surrounding intraparenchymal brain tumours, and more specifically, to define epileptogenic brain tissue in patients with pharmacoresistant epileptic seizures (Bancaud et al., 1975). The approach required a hypothesis that would enable the placement of multiple, mostly unilateral, depth electrodes to sample a reasonably limited number of regions of interest. The results then guided a tailored surgical resection. This technique was adapted by Crandall et al. (1963) at UCLA for patients with suspected mesial temporal lobe epilepsy. Crandall,