* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download hematopoietic stem cells: to be or notch to be
Survey
Document related concepts
Hedgehog signaling pathway wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Transcript
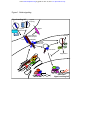
From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Blood First Edition Paper, prepublished online February 3, 2012; DOI 10.1182/blood-2011-10-355826 HEMATOPOIETIC STEM CELLS: TO BE OR NOTCH TO BE Anna Bigas and Lluis Espinosa Program in Cancer Research. Institut Mar Investigacions Mèdiques (IMIM), Hospital del Mar, Parc de Recerca Biomèdica de Barcelona, Barcelona, Spain. Corresponding author: Anna Bigas IMIM-Hospital del Mar Dr. Aiguader 88 08003 Barcelona Phone: +34 933160440 Fax: +34 9321600410 e-mail:[email protected] Copyright © 2012 American Society of Hematology From www.bloodjournal.org by guest on June 14, 2017. For personal use only. SUMMARY Notch is a well-conserved signaling pathway and its function in cell fate determination is crucial in embryonic development and in the maintenance of tissue homeostasis during adult life. Notch activation depends on cell-cell interactions that are essential for the generation of cell diversity from initially equivalent cell populations. In the adult hematopoiesis, Notch is undoubtedly a very efficient promoter of T-cell differentiation and this has masked for a long time the effects of Notch on other blood lineages, which are gradually being identified. However the adult Hematopoietic Stem Cell (HSC) remains mostly refractory to Notch intervention in experimental systems. In contrast, Notch is essential for the generation of the HSCs, which takes place during embryonic development. This review summarizes the knowledge accumulated in the recent years regarding the role of the Notch pathway in the different stages of HSC ontology from embryonic life to fetal and adult bone marrow stem cells. In addition, we will briefly examine other systems where Notch regulates specific stem cell capacities, in an attempt to understand how Notch functions in Stem Cell Biology. INTRODUCTION Stem Cell Biology is dependent on a few signaling pathways that crosstalk each other to generate a network of biochemical interactions and are ultimately translated into precise instructions at the nuclear level. The Notch pathway has been evolutionary preserved not only biochemically but also functionally, being involved in establishing cell diversification from equipotent adjacent cells using a mechanism that requires cell-cell interaction. Essentially, Notch-mediated communication depends on the differential expression of specific ligands (Jagged or Delta) and receptors (Notch1-4) in adjacent cells. Basically, Notch signaling is transduced from the sending cell (the one that expresses higher levels of the ligand) to the nucleus of the receiving cell (expressing higher levels of Notch receptors) thus impinging on its biochemical network 1. Cell-cell interactions are essential players in the regulation of Stem Cell and tissue homeostasis of the adult but also in developing organisms. In general, the tag “stem” refers to cells with unlimited capacity for self renewal and with the ability to generate all the different lineages of a specific system. However, when studying stem cells one need to distinguish two main categories of stem cells: 1) embryonic stem cells which are pluripotent and retain the capacity to generate all the cell lineages of the adult organism and 2) somatic stem cells which are also generated in the developing embryo, maintain the self renewal capacity, but show a reduced pluripotency since they can 2 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. only generate a limited number of cell types. The latter include the ones involved in tissue formation and regeneration both in the embryo and the adult 2 The hematopoietic system has served for decades as a pioneer model for studying and deciphering the behavior of somatic Stem Cells and the mechanisms that regulate cell differentiation, and in fact, the knowledge generated on hematopoietic stem cell biology has guided the understanding of other types of somatic stem cells. From these studies, the Notch pathway has emerged as a principal player on stem cell regulation and differentiation even though many questions on how or whether Notch functions in hematopoietic stem cells remain still unanswered. Interestingly, studies in the adult hematopoietic stem cells first unexpectedly revealed that the maintenance of this type of stem cells was independent of Notch. However, further understanding of the ontogeny of HSC has now provided new insights about the predominant role of Notch in the generation of HSC in the embryo. The aim of this review is to gain a better understanding of the Notch pathway and integrate the results obtained in the last decades by analyzing HSC from the adult bone marrow and the mouse embryo. Some Notch History Notch is a well-conserved signaling pathway that was first identified in Drosophila mutants. Lack of the X-linked Notch locus in the fly resulted in embryonic death, yet heterozygous females showed the serrate/notched wing margin first observed by T.H. Morgan’s group 3, a phenotype that named the whole pathway. Drosophila 4,5 The Notch gene was initially cloned in and soon after in Caenorhabditis elegans 6,7 , Xenopus8, mouse9 and human10. In general, Notch works as a determinant factor during binary cell decisions from adjacent cells, a function that was elegantly demonstrated in C. elegans11 and Drosophila12. Later on, the sequence of the first Notch ligands was obtained and the analysis of the proteins indicated that they all corresponded to cell surface proteins with a large extracellular domain, which further supported a function for Notch as a regulator of cell-cell interactions between neighboring cells. Deciphering the elements downstream of the receptor and ligands involved laborious work from embryologists and biochemists that studied and interpreted the results from different mutant models. Thus, genetic experiments in invertebrates first directed the investigations of the Notch pathway towards the identification of Supressor of Hairless (Su(H)) as a key transducer of neurogenic signals13,14. Further biochemical studies demonstrated the interaction between Notch and Su(H), the orthologue of the mammalian gene Rbpj (for Recombination-signal Binding Protein jk)15. Genetic screenings were also crucial to identify the Notch-target genes as Hairy and Enhancer of Split (Hes), which are HLH (Helix loop Helix) proteins involved in 3 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. suppressing the neuronal genes Acute-Scute16,17. Once again, this connection is conserved in the mammalian systems. Later on, efforts were focused on investigating Notch signal transduction. The demonstration that Notch together with Su(H)/RBP-J regulated the transcription of Hes genes14,18 provided a strong indication that Notch should function in the nucleus. In fact, it was initially shown that ectopically expressed intracellular Notch translocated to the nucleus and was capable of activating transcription19 but ingenious experiments were required to demonstrate the nuclear activity of endogenous Notch20. Another breakthrough discovery in the history of the Notch field was the demonstration that this receptor was processed by a γ-secretase/presenilin complex in response to ligand binding, and the consequent development of inhibitors that target this activity 21 . The usage of these Notch/γ-secretase inhibitors has facilitated the further confirmation of most Notch functions in different cell types and tissues and provided the first attempts of using Notch as a therapeutical target 22,23 . During the last decade, research in the Notch field has been growing exponentially, which has contributed to a better understanding of the relevance of this pathway in the generation and maintenance of multicellular organisms. Elements of the Notch signaling pathway Notch is a single-pass transmembrane receptor that can be activated by different transmembrane ligands. In general, Notch binds to one of its ligands located in the adjacent cell, which results in two sequential proteolytic cleavages of the receptor. The latter involves the release of the intracellular part of Notch receptor (IC-Notch), its translocation to the nucleus, and its association with RBP-J (also known as CSL for the different orthologue proteins: CBF1, Supressor of Hairless and Lag1) and the co-activator Mastermind (Mam) to activate specific transcription. Notch participates in the transcriptional regulation of multiple genes, some of them being context-dependent. However, the most important Notch functions have been associated with the regulation of the Hes or Hes-related (Hrt) family of genes. All these genes encode for bHLH proteins that, in general, function as inhibitors of cell differentiation. Notch receptors There is one Notch receptor in Drosophila, two in C. elegans and four different Notch receptors (Notch 1-4) in most vertebrate species, being mammalian Notch1 and Notch2 the most similar to the Drosophila homologue. Notch is a single transmembrane protein composed of an extracellular part with variable number of Epidermal Growth Factor (EGF)like repeats and an intracellular part containing 7 ankyrin-like repeats, nuclear localization 4 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. signals and a transactivation domain. Notch is codified by a single mRNA molecule that translates into a polypeptide, which is cleaved in the Golgi apparatus by a furin-like convertase enzyme24. This processing generates two different fragments (one containing the extracellular domain and another that includes de transmembrane and intracellular domains) that remain associated by disulfide bonds involving a small conserved extracellular domain, LNR (Lin/Notch repeats)25. Hence, the resulting functional Notch is commonly considered as a “heterodimeric” receptor. The LNR region is also crucial to prevent ligand-independent signaling, which is supported by the fact that mutations in this region results in increased Notch activity that associates with T-ALL leukemia26. Notch ligands There are at least 5 functional Notch ligands in vertebrates: three orthologues of the Drosophila Delta (Delta or Delta-like (Dll) 1, 3 and 4) and two of the Drosophila Serrate (Jagged1 and Jagged2). All ligands are able to interact with the Notch receptor and induce the second cleavage at the extracellular level. However, all ligands have different expression patterns and specific deletion/inhibition of specific ligands results in a very diverse outcome. Notch ligands are also composed by a variable number of EGF-like repeats in their extracellular domain but a small intracellular portion. To achieve Notch activation, Notch ligands need to be internalized in endosomes in the signaling sending cell (before they are presented at the cellular membrane), a process that is regulated through ubiquitination by the E3-ubiquitin ligases Mindbomb and Neuralized 27. The consequence of Mindbomb deficiency is a defective Notch activation, in both invertebrates and vertebrates28,29. Notch modification by glycosylation: pofuts, fringes and pogluts One of the particularities of Notch is the absence of a downstream signaling cascade. Instead, following Notch activation IC-Notch travels from the cell surface directly to the nucleus, where it binds RBP-J and indirectly to the DNA. Despite the apparent simplicity of this pathway with no intermediate effectors, Notch signaling involves an extremely accurate regulation, which is multifactorially achieved. For example, multiple enzymes can modify the Notch protein post-translationally, thus changing its functional properties. Pofut-1 is an Ofucosyl-transferase that catalyzes the O-fucosylation of specific EGF-like repeats, which is an essential condition for their subsequent Fringe-dependent modification. Fringe proteins (including Lunatic, Manic and Radical Fringe) are Golgi-localized glycosyltransferases that add N-acetylglucosamine to O-fucose moieties on EGF-like repeats of the extracellular domains of Notch. Different Fringe homologues modify specific EGF-like repeats with distinct efficiencies 30 . Fringe-modifications enhance the capacity of Notch to be activated 5 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. by ligands of the Delta-like family (Dll1,3 and 4) whereas reduce Notch activation by the Serrate/Jagged family of ligands (Jag1-2)31. While some studies suggest that knockout mutants for all three Fringes (in a specific genetic background) do not display more developmental malformations than single mutants for Lunatic Fringe 32, it is well established that different Fringe homologues modulate particular Notch-mediated biological processes in a specific manner 33-35 , such is the case of Lunatic Fringe in the hematopoietic lineage commitment and differentiation 36-39. Another modification involving the extracellular domain of the Notch receptor is its Oglucosylation mediated by the O-glucosyl-transferase (Poglut), Rumi 40. However, which are the biochemical effects of this modification is mainly unknown but it has been suggested that it might regulate the proper folding of Notch that is required for its efficient activation. Downstream effectors of Notch: The Hes gene family Hes genes and specifically Hes1 are among the best-characterized Notch target genes. They codify for bHLH proteins and, in general, function as DNA-binding transcriptional repressors. Expression of several members of the family (including Hes1, Hes5, Hes7, Hrt1 and Hrt2) depends mostly on Notch activity and participate in many of the Notch-assigned functions including proliferation, differentiation, apoptosis, self-renewal and asymmetric cell division regulation (reviewed in 41). Hes genes are generally responsible for Notch functions that require the inhibition of one specific cell fate to allow the determination of an alternative fate (lateral inhibition) whereas other Notch inductive functions such as T-cell specification may not be dependent on Hes. In this sense, Hes1 is only needed for the first stages of TCell determination42 while other important Notch targets such as pTα43, IL7R44 are expressed and regulate specific stages of T-Cell differentiation. Embryonic development and Somatic Stem Cell generation During embryonic development, specification of the different tissues runs in parallel with the progressive restriction of the stem cell potential. However, significant pools of stem cells are found in the adult tissues that are continuously renewed during lifetime, such as blood or intestine. For many years, hematopoietic stem cells remained the best-characterized somatic stem cells and, consequently, they have been used as a model to study adult tissue regeneration. In the recent years, similar types of cells have been identified in many other tissues such as muscle, neuronal, pancreas, lung and breast, among others and it is now widely accepted that they are crucial players in tissue homeostasis and regeneration. In the blood system, there is now definitive evidence that the somatic-, blood-specific stem cells are originated during embryonic life and maintained thereafter. Although Notch is generally considered as an essential signaling pathway that regulates multiple stem cell 6 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. functions, its participation diverges among the different organs and tissues. For example, in the mammalian hematopoietic system Notch1 is required for the generation of hematopoietic stem cells in the embryo but it is dispensable for the maintenance of the hematopoietic stem cells in the adult organism (reviewed in 45), whereas the neuronal stem cells depends on Notch in both embryonic and adult life. Our current understanding on how Notch contributes to tissue homeostasis includes a plethora of functions in both the stem and progenitor cell populations that cannot be generalized from one tissue to the others. In this review, we will summarize what is known about Notch functions during generation and maintenance of HSC, and compare this with its role in other tissue-specific stem cell populations. Notch in Hematopoietic Stem Cells: Generation and Maintenance Multiple types of specialized cells, which are responsible for nutrient transport and immunedefense, constitute the hematopoietic system. During the adult life, all hematopoietic cells in the organism are renewed every 5 to 120 days depending on the cell type. Continuous production of limited numbers of blood cells is achieved by the differentiation of Hematopoietic Stem Cells in the bone marrow microenvironment. However, it is the exclusive capacity of HSC for self-renewal that guarantees the maintenance of this tissue throughout life. The question that arises here is, how this rare population acquires the features of self-renewal and pluripotency that define a stem cell. From our knowledge stem cell properties are acquired during embryonic life, thus in the following sections we will review what it is known about the mechanisms that regulate the generation and maintenance of HSCs and the role of Notch in these different processes. Embryonic hematopoiesis Before colonizing the bone marrow, newly formed HSCs as well as all different hematopoietic lineages are found in the developing vertebrates in a tightly-regulated spatial and temporal manner. More specifically, embryonic hematopoiesis takes place in two different waves namely primitive and permanent-definitive hematopoiesis (reviewed in46). In mammals, primitive hematopoiesis occurs primarily in the blood islands of the yolk sac47,48 around murine embryonic day 7.5 (E7.5) 49, and whether Yolk Sac blood cells originate from a common endothelial progenitor called hemangioblast50,51 is still unclear48. Grafting experiments using quail-chick chimeras and Colony Forming Unit (CFU) cultures showed that the yolk sac mainly produces primitive erythrocytes and myeloid progenitors but does not generate definitive HSCs 52-56 . In fact, HSCs are first detected in the yolk sac after circulation between the yolk sac and the embryo is established (in the mouse embryo HSC are found at E9 and circulation starts around E8.5) 57 . Placenta is also an important source 7 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. of erythroid and myeloid progenitors at E9.0 and interestingly it becomes a reservoir of HSCs around E1258-60. However, the first definitive HSCs considering those cells with capacity to reconstitute irradiated adult mice appear in the Dorsal Aorta, in a region surrounded by Gonad and Mesonephros (also known as AGM region) around day 9 of murine development61,62, closely associated with the endothelial cells. Cell-tracing experiments demonstrated that murine adult hematopoietic cells originate from embryonic cells that express VE-cadherin and Runx1 around E9.0, strongly suggesting the endothelial embryonic origin of HSCs but not excluding other possibilities 63,64 . Importantly, endothelial cells from fetal liver are no longer capable of generating hematopoietic cells indicating that a putative common endothelial-hematopoietic progenitor should be very restricted both temporally and spatially to specific embryonic vessels, such as the dorsal aorta 65. Notch in embryonic arterial and hematopoietic development One of the best-characterized Notch functions in vertebrates consists in the determination of the arterial program, and several Notch mutant embryos in both zebrafish and mouse lack artery specification as determined by the absence of EphrinB2, CD44 or SMA expression 66-68 . Accordingly, since HSC originates in arterial vessels the predicted phenotype of Notch mutant embryos was indeed lack of hematopoiesis. This is exactly the phenotype of the Notch1, Rbpj and Mindbomb deficient mutant mice 69-71 . In contrast, coup- TFII mutants show ectopic Notch activation in veins accompanied by the expression of 72 arterial markers and ectopic hematopoiesis . Other Notch mutants that fail to define the artery-vein boundaries such as Activin A-receptor typeII-like1 (acvrl1, alk1) or endoglin (CD150) show ectopic hematopoiesis in veins or at least in vein-like vessels that do not express the arterial marker EphrinB2 72-74 . This link between arterial specification and hematopoiesis has complicated a direct assignment of the hematopoietic defects to Notch deficiency, since secondary effects of arterial development may result in the same phenotype. Opportunely, experiments in zebrafish showed that ectopic Notch1 activation in the veins induced specific hematopoietic gene expression including Runx1 and c-myb in the absence of arterial differentiation 71. However, a formal proof that HSC can be generated in the absence of arteries is still missing. Further insight into the question on how Notch regulates hematopoiesis independently of its role on arterial specification was obtained by the analysis of the Jagged1 mutant. In this work, we demonstrated that Jagged1, Jagged2 and Delta4 are all expressed in the endothelium of the aorta in the developing AGM 70 . However, analysis of the mutants demonstrated that they do not display redundant functions. Specifically, Delta4 deficient mice show a strong arterial phenotype at early developmental time. Similarly, malformations 75 Jagged1 mutants show 67,68 and die some vascular but still express arterial markers such as EphrinB2, CD44 and SMA and 8 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. importantly they mostly failed to generate hematopoietic cells 76 . These results undoubtedly demonstrated that Notch was required for definitive hematopoiesis in vivo, and uncouple this Notch function from its role in arterial specification. Another unexpected result that arose from the analysis of mutant mice was the fact that only definitive hematopoiesis in the AGM region, but not the embryonic hematopoiesis of the yolk sac, was Notch-dependent. As discussed above murine yolk sac from E7.5 to E8.5 mainly produces primitive nucleated erythrocytes (an event known as primitive erythropoiesis) and it is not until E9.0 when circulation is already established that the yolk sac contain different hematotopoietic progenitors capable of generating myeloid, erythroid or lineage-mixed colonies in culture, but also definitive HSCs that are transplantable and able to reconstitute busulphan-treated newborn mice. Importantly, the yolk sac from Notchdeficient mutants contains similar numbers of hematopoietic progenitors at different times of development compared to their wild type or heterozygous counterparts HSC potential at E9.0 (15-20 somites) 69,77 but lacks the 69 . Together these results indicate that Notch signaling is dispensable for primitive hematopoiesis whereas, independently of the site of origin (provided that there is more than one), Notch signaling is essential for generating definitive hematopoiesis. In agreement with this idea, analysis of chimeric mice generated from wildtype and Notch1-deficient ES cells demonstrated that Notch1-deficient cells contribute to all types of embryonic, fetal progenitors and hematopoietic cells present in fetal liver, but do not contribute to adult hematopoiesis 78 . Similarly, mutation of Mindbomb in zebrafish affected the later wave of hematopoietic cells (characterized by expression of c-myb or Runx1) but not for the early phase of primitive cells 79. Notch in Fetal hematopoiesis The final purpose of developing HSCs is to access their niche in the bone marrow that will allow HSC self-renewal and differentiation throughout the adult life. However, before reaching the bone marrow newly formed HSCs migrate to the fetal liver, which is the main hematopoietic organ before birth. During this period, early lymphoid fetal liver progenitors need to colonize the thymic epithelial primordium where they experienced high Notch signaling dose and differentiate into T-cells 80 , whereas HSC will need to colonize the bone marrow. However, the most remarkable hematopoietic process in the fetal liver is that HSCs increase in number. For this reason fetal liver signals have been studied for many years in an attempt to identify candidate molecules that prone HSC expansion. Whether Notch participates in this particular fetal process is mainly unknown basically because most of conventional Notch mutants are either lethal or failed to generate hematopoiesis before the fetal liver stage. Thus, analysis of conditional Notch mutants that specifically affect the fetal liver stage may be useful to uncover any possible role of Notch in this process. 9 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Notch in Adult Hematopoietic Stem Cells Early studies demonstrated that Notch is expressed in the undifferentiated progenitors from human bone marrow cells 81 and suggested a putative role for this factor in leukemia 10 . Later on, experiments using myeloid progenitor cell lines indicated that Notch is an essential regulator of hematopoietic differentiation 82,83 , thus opening the possibility that it might function in preserving the stem cell phenotype. In addition, Parathyroid Hormone that is a crucial regulator of the osteoblastic HSC niche in the bone marrow, increases the levels of Jagged1 in this tissue with in vitro evidences of Notch function in HSC expansion84. However, the analysis of transgenic mice carrying a dominant negative form of the coactivator Mastermind (that specifically blocks all canonical Notch signaling) or mice deficient for Rbpj indicated that Notch activity was dispensable for the maintenance of HSC in the adult bone marrow under physiological conditions 85 . Further support for these results can be found in the conditional deletion of Notch1 or Notch1 plus Notch2 under the control of the interferon-dependent expression of Mx-Cre that specifically and exclusively affected lymphoid differentiation, but not other hematopoietic lineages86-88. Consistent with the notion that Notch activity is dispensable in the adult HSC a recently published database (www.immgen.org)89 showed low levels of different Notch receptors in purified HSCs. Notch-dose dependency in hematopoietic differentiation and hematopoietic disorders Experimental systems that mostly rely on complete abrogation or strong persistent activation of the Notch pathway result in phenotypes that are mainly associated with the lymphoid lineage consistent with the fact that high Notch activity promotes T-cell differentiation whereas complete absence of Notch induces early B-cell commitment 86,90 . In agreement with this, activating Notch mutations are commonly found in T-cell leukemias (for review see91,92). However, whole genome sequencing of B-chronic lymphocytic leukemia (B-CLL) cells has revealed that 12% of these samples also contain activating mutations of Notch associated with a Notch-active signature 93 , suggestive of a regulatory role for Notch in B-cell differentiation which is also in agreement with the effects of Rbpj or Notch2 deletion in committed B-cell progenitors that impaired Marginal Zone B-cells and potentiates follicular B-cell differentiation in the spleen94,95. Unexpectedly, the identified activating mutations in B-CLL affect the Notch1 receptor, which is not the main effector for B-cell terminal differentiation, suggesting that aberrant transforming Notch activity can be delivered by any receptor. In fact, this emphasizes the importance of large-scale genome sequencing of patients with different hematopoietic disorders in providing a new perspective about the involvement of Notch in these malignancies. The knowledge on how much, when and where Notch is required to maintain the correct specification of the hematopoietic 10 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. lineages (including the HSCs), is an essential requirement for further manipulation of the Notch pathway for biomedical purposes. However, rather than just being a Notch ON/OFF issue the extent of Notch activity is also critical for most of the Notch functions involved in the homeostasis of the hematopoietic lineages. Alternative approaches that overcome this limitation include the intervention on positive or negative regulators, which might affect the strength of Notch signal. Indeed, overexpression of Lunatic fringe that reduces the affinity between Notch and delta ligands forces lymphoid progenitors to become B-cells in a high delta presenting environment39 whereas modulating the density of Delta1 influences the generation of B or T cells in vitro96. Recent works describing the phenotype associated with loss-of-function mutations of positive Notch regulators such as Mindbomb 97 , Nicastrin 98 , Presenilin99 or Pofut 100 suggested that reduction in the levels of Notch activity is sufficient to induce a myeloproliferative syndrome that phenocopies the effects observed in one of the Mx-Cremediated Notch1 and Notch2 deletion98, despite that the same experimental model was previously generated showing an exclusive lymphoid phenotype88. Consistent with the possibility that myeloid differentiation/proliferation is favored by reduced Notch activity (see model in Figure 2), loss-of-function mutations of the pathway are present in about 12% of patients with chronic myelomonocitic leukemia (CMML)98. Interestingly, mouse transplanted with hematopoietic cells in which RBPJ-mediated Notch activity is completely abrogated including Rbpj-deletion or ectopic DN-Mam expression85 did not show any myeloproliferative phenotype, suggesting that RBPJ-independent or non-cell-autonomous Notch activities are required to develop the disease. In addition, a different study showed that expression of DN-Mam precludes Megakaryocyte differentiation in vivo101. On the other hand, most of the phenotypes associated to Notch pathway manipulation suggest an almost exclusive cell-autonomous requirement for Notch signaling in the maintenance of the hematopoietic system. However, mice deficient for Pofut1, which is required upstream of Fringe glycosyltransferases to permit Notch signaling through Delta, displayed both cell-autonomous and non-cell-autonomous defects that resulted in myeloproliferative disease100 suggestive of undetermined Notch functions in the regulation of the stromal HSC niche. In addition, defective Notch signaling in the murine skin results in an epidermal-barrier-associated inflammatory phenotype, which leads to massive Thymic Stroma Lymphopoietin (TSLP) expression and produces lethal B-cell102 or myeloid proliferative disorders103. In conclusion, and to help understand how Notch impinges in the hierarchy of hematopoietic lineages, we propose a Notch-dose-based model that is compatible with recent data and revised models104-107 in which specific Notch activity levels impose progressive lineage restrictions (see model in Figure 2). 11 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Notch in ex-vivo hematopoietic cultures and stress conditions Nowadays there is no evidence for a physiological role of Notch in the maintenance of HSC in vivo. Despite this fact, I.D. Bernstein’s laboratory has recently demonstrated that Notch pathway manipulation is a potential way of expanding HSCs or progenitor cells for clinical applications. The basis for this finding comes from early experiments in which murine undifferentiated bone marrow cells (Lin-Sca+Kit+) were transduced with retrovirus encoding active Notch1. In this pioneer experiment, cells with self-renewal and pluripotent myeloid and lymphoid differentiation capacity in vitro and in vivo were obtained 108 . To avoid using retrovirally-infected cells, they have now optimized the culture conditions to activate endogenous Notch through immobilized Notch ligands109. This strategy turned out to be particularly efficient for the expansion of short-term lymphoid and myeloid progenitors from samples with low numbers of these cell populations such as cord blood. This strategy is now under clinical investigation in immune-ablated patients, which are being transplanted with one un-manipulated plus one Notch-expanded cord blood units. The first evaluated patients have significantly reduced the time of neutrophil engraftment compared with patients transplanted with two un-manipulated cord blood units110. However, Notchdependent expansion protocols can still be improved to enhance the long-term engraftment of the manipulated unit after the identification of crucial elements provided by endothelial cells111 or by using different ligands112. At the mechanistic level, the impact of Notch activation on ex-vivo expansion of LT- or ST-HSC may be directly correlated with its capacity to regulate cell cycle in HSC113 or/and hematopoietic recovery under stress conditions87. What can we learn from other systems: Notch function in other somatic stem cells Different Notch functions that take place in particular cell types, tissues or developmental stages involve specific mechanistic strategies that are under intense investigation. For example, many efforts have been made to understand how Notch regulates stem cell maintenance and cell fate determination in both embryonic and regenerating adult tissues. Despite some of the identified mechanistic strategies can be tissue specific, others maybe not, or in any case they can serve as inspiration for people working on different systems (see Figure 3). In the next section we have selected some of these examples, which may apply to the hematopoietic system. The oscillation model: Neural Stem Cells Similar to HSC, neural stem cell generation also occurs during embryonic life. However, some specific regions of the adult hippocampus (subgranular zone, SGZ), lateral ventricle 12 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. (subventricular zone, SVZ) and the olfactory bulb still contain some functional neural cells with self-renewal capacity. During embryonic development, different phases of both symmetric and asymmetric cell divisions are required to generate all main neuron lineages: First, symmetric divisions of the neural stem cells support the expansion of the neuroepithelial progenitors and the generation of radial glial cells. Next, asymmetric division of these progenitors gives rise to all types of cells during the neurogenic and gliogenic phases. It is well established that Notch pathway is a crucial regulator of this process and it is required to maintain neuronal progenitors and inhibit neuronal differentiation (for review see 114 ). A nice example on how Notch is differentially activated in the developing neurons is found during asymmetric division of cortical neuronal progenitors. In this process, both the Notch receptor and its inhibitor Numb are asymmetrically distributed in the two daughter cells. The one carrying Notch induces hes1 transcription that directly inhibits neuronal genes. However, the system is not so simple since Hes1 protein inhibits its own transcription thus generating a negative feedback loop that creates an oscillatory pattern of hes1 expression 115 . Oscillations on Hes1 protein levels induce the oscillation of its target genes including Neurogenin2 (Ngn2), but also Delta1 (Dll1), which in turn induces oscillation of Notch activity in neighboring cells 116 . Elegant studies with time-lapse imaging from Kageyama’s laboratory showed that Ngn2 and Dll1 oscillate in neural stem/progenitors in an opposite phase as hes1, and their expression is sustained in differentiating neurons where Hes1 expression is repressed (reviewed in 117 . This oscillatory system is responsible for maintaining active proliferation and neuronal determination in the developing midbrain, whereas sustained Hes1 levels in the cells of the boundary of the forebrain, midbrain and hindbrain maintain low levels of Ngn2 that result in low proliferation and prevent neuronal formation. In the adult brain, neurogenesis is much more restricted in the number and differentiation potential of cells undergoing this process. However, Notch seems to be also involved in the maintenance of the adult neural stem cell population, whereas inactivation of Notch signaling induces neuronal differentiation and depletes the neural stem cell pool 118,119 Cell versus non-Cell autonomous or embryonic versus adult Notch effects: Skin Stem Cells The adult skin epidermis contains two different types of progenitor or stem cells: The proliferative cell compartment that is located in the basal layer of the interfolicullar skin and can differentiate into a single lineage and the mostly quiescent multipotent stem cell compartment restricted to the bulge of the hair follicle. The bulge stem cells (that are the pluripotent skin stem cells) can generate both epidermal and hair follicle cells dependent on Notch activity, since Notch-RPP-J signaling promotes the follicular fate 120,121. The activation of Notch in this system is downstream of β-catenin activity through the transcriptional 13 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. activation of Jagged1 122 . However, there is no evidence for Notch function in maintaining the multipotent bulge stem cell compartment. In the basal epidermal layer, Notch signaling regulates proliferation and differentiation of the epidermal cells, however some data is still controversial and mainly depends on the experimental systems used. Early studies using epidermis-specific Notch1 knock out mice (using Keratin5-Cre) demonstrated that Notch deletion increased skin proliferation by downregulating p21 in the keratinocytes increased β-catenin levels 124 123 thus creating a tumor-promoting environment with . These findings, together with data from knock-down and knockout of different Notch elements in the mouse skin supported the idea that Notch behaved as a tumor-suppressor in this tissue 125 . Surprisingly, Rbpj-deletion in the developing skin (by using the keratin14-CRE mouse line) resulted in a hypo-proliferative phenotype concomitant with a blockage in skin differentiation, that was non-cell autonomous since it was recovered when mutant skin was grafted onto nude mice 126 . These results, further than creating controversy, highlighted the great complexity of the interactions that mediate Notch effects and suggested that non-cell autonomous effects might also influence the effect of Notch in skin tumorigenesis. In fact, using a mouse model with chimeric pattern of Notch1-deletion in the skin it was recently demonstrated that it is not the absence of Notch in the keratinocyte progenitors but the skin barrier defects induced by Notch deletion, what is responsible for tumor promotion in these mutants Analysis of mice that are mutant for the Notch-processing enzymes γ-secretase Adam10 128 127 . and 129 , have further confirmed that Notch plays both cell autonomous and non-cell autonomous effects in the adult skin. In the embryo, deletion of Rbpj prevented the transition between basal to spinous layers 126 , whereas Adam10 mutants showed a premature differentiation from spinous to granular layers 129 , which in both cases generates and aberrantly thin spinous layer. Of note, embryonic inactivation of γ-secretase did not impose any phenotype to the developing skin. The niche-forming Stem Cell model: Intestinal Stem Cells The intestinal epithelium is one of the most rapidly self-renewing tissues of the organism (every 4-5 days for most cell types). In the adult, all post-mitotic cell lineages are originated by terminal differentiation of a progenitor pool derived from the intestinal stem cells (ISC). Few years ago, it was proposed that a highly proliferative population of cells residing in the bottom of the crypts, characterized by expression of the Lgr5 marker, were the actual ISC. Importantly, these cells reside adjacent to terminally differentiated Paneth cells that support their self-renewal and pluripotent capacities by providing Wnt and Notch-ligands 130. Recently, it has been shown that Lgr5-positive cells can derive from quiescent Bmi1 expressing cells located in the +4 position (relative to the bottom of the crypt) 131 , which 14 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. suggest the co-existence of more than one ISC compartment (for review see 132 . Notch plays an essential role in the maintenance of intestinal homeostasis, since inhibiting Notch, both pharmacologically and genetically, impairs the integrity of the intestinal crypt leading to the accumulation of post-mitotic goblet (mucus-secreting) cells 22,133 . However, this effect is supposed to be dependent on Hes1, which inhibits Atonal that is required for differentiation into the secretory lineage intestine 135 134 . Notch is also expressed in the stem cell compartment of the and mice carrying specific intestinal deletion of Delta1-4 ligands failed to activate Notch, lacking the expression of stem cell markers in the crypt cells (including Lgr5 and Olfm4) 136 . This fact suggests a role for Notch not only in regulating intestinal differentiation but also in the maintenance of the ISC. However, the fact that secretory Paneth cells (that depends on Atonal, which is inhibited downstream of Notch) participate of the intestinal stem cell niche precludes giving a definitive answer about whether Notch directly regulates intestinal stemness or it is just an indirect effect due to possible Paneth cell number alterations. However, in a simplistic point of view Notch inhibition should lead to increased Paneth cell numbers and favors ISC generation, which is not the case. The role of Notch in regulating the niche for stem cell generation or maintenance is a novel concept, with emerging evidences not only in the mammalian intestinal system but also in the development of the drosophila gut 137 as well as the hematopoietic development of zebrafish 138, which should be investigated in the near future. A regenerative medicine hallmark: myogenesis. Finally, another important function of Notch is as a regulator of myogenesis both during embryonic somitogenesis and in post-natal muscle repair. Importantly, inducing Notch signaling by Delta1 potentiate muscle regeneration in old aged mice. Specifically, the balance between Notch and TGFb/smad3 signals determine the levels of CDK inhibitors that are crucial regulators of the proliferative capacity of muscle progenitors139. Similarly, activation of Notch in satellite cells blocks myogenesis whereas inhibition of the pathway leads to increased cell differentiation, indicating that Notch signal is essential to regulate the pool of satellite cells and adult tissue repair (reviewed in 140). CONCLUSIONS AND PERSPECTIVES One of the take home messages of this review is the essential role of Notch in the regulation of most binary cellular decisions that take place both in the embryo and in adultregenerating tissues, which is exemplified in the hematopoietic system. It is thus envisioned that the possibility to modulate Notch signaling will open an avenue for regenerative medicine applications, including hematological and non-hematological diseases, but also for cancer treatment. Before that, much more detailed studies including mechanistic data 15 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. from hematopoiesis but also other embryonic and adult tissues are required to clearly determine whether and how Notch can be used as a tool for generating clinically relevant and safe cell populations for transplantation. ACKNOWLEDGEMENTS We sincerely apologize to those whose work could not be cited due to space limitation. We thank the members of the Bigas-Espinosa lab for critical discussions, specially to Teresa D’Altri, Leonor Norton, Julia Inglés-Esteve and Erika López-Arribillaga for critical reading of the manuscript. The laboratory is funded by Ministerio Ciencia e Innovación (SAF200760080, PLE2009-0111, SAF2010-15450, PI10/01128), Red Temática de Investigación Cooperativa en Cáncer (RTICC) (RD06/0020/0098), AGAUR (2009SGR-23 and CONES2010-0006. LE is an investigator of ISCIII program (02/30279). Authorship: L.E. and A.B. wrote the review. Conflict of Interest: No financial conflicts of interest apply. REFERENCES 1. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770-776. 2. Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367-409. 3. Morgan TH. The Theory of the Gene. The American Naturalist. 1917;51(609):513. 4. Artavanis-Tsakonas S, Muskavitch MA, Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc.Natl.Acad.Sci.U.S.A. 1983;80:1977-1981. 5. Kidd S, Lockett TJ, Young MW. The Notch locus of Drosophila melanogaster. Cell. Sep 1983;34(2):421-433. 6. Yochem J, Weston K, Greenwald I. The caenorhabditis elegans lin-12 gene encodes a transmembrane protein with overall similarity to drosophila notch. Nature. 1988(6190):547-550. 7. Yochem J, Greenwald I. glp-1 and lin-12, genes implicated in distinct cell-cell interactions in C. elegans, encode similar transmembrane proteins. Cell. Aug 11 1989;58(3):553-563. 8. Coffman C, Harris W, Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990;249:1438-1441. 9. del Amo FF, Smith DE, Swiatek PJ, et al. Expression pattern of Motch, a mouse homolog of Drosophila Notch, suggests an important role in early postimplantation mouse development. Development. 1992;115(3):737-744. 16 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 10. Ellisen LW, Bird J, West DC, et al. TAN-1, the Human Homolog of the Drosophila Notch Gene, Is Broken by Chromosomal Translocations in T Lymphoblastic Neoplasms. Cell. 1991;66:649-661. 11. Seydoux G, Greenwald I. Cell autonomy of lin-12 function in a cell fate decision in C. elegans. Cell. 1989;57:1237-1245. 12. Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. Dec 1985;43(3 Pt 2):567-581. 13. Schweisguth F, Posakony JW. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. Jun 26 1992;69(7):1199-1212. 14. Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. Oct 21 1994;79(2):273-282. 15. Matsunami N, Hamaguchi Y, Yamamoto Y, et al. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature. Dec 21-28 1989;342(6252):934-937. 16. Bailey AM, Posakony JW. Suppressor of Hairless directly activates transcription of Enhancer of Split complex genes in response to Notch receptor activity. Genes Dev. 1995;9(21):2609-2622. 17. Vassin H, Vielmetter J, Campos-Ortega JA. Genetic interactions in early neurogenesis of Drosophila melanogaster. J Neurogenet. Nov 1985;2(5):291-308. 18. Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377(6547):355-358. 19. Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated notch receptor blocks cell-fate commitment in the developing drosophila eye. Nature. 1993;365(6446):555-557. 20. Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382-386. 21. De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gammasecretase-like protease mediates release of Notch intracellular domain. Nature. Apr 8 1999;398(6727):518-522. 22. van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. Jun 16 2005;435(7044):959-963. 23. Real PJ, Tosello V, Palomero T, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. Jan 2009;15(1):50-58. 17 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 24. Logeat F, Bessia C, Brou C, et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Procedings of the National Academy of Science. 1998;95(7):8108-8112. 25. Blaumueller CM, Qi H, Zagouras P, Artavanis Tsakonas S. Intracellular Cleavage of Notch Leads to a Heterodimeric Receptor on the Plasma Membrane. Cell. 1997;90(2):281-291. 26. Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. Oct 8 2004;306(5694):269-271. 27. Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. Apr 2005;3(4):e96. 28. Itoh M, Kim CH, Palardy G, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. Jan 2003;4(1):67-82. 29. Koo BK, Lim HS, Song R, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. Aug 2005;132(15):3459-3470. 30. Wang Y, Shao L, Shi S, et al. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein Ofucosyltransferase. J Biol Chem. Oct 26 2001;276(43):40338-40345. 31. Yang LT, Nichols JT, Yao C, Manilay JO, Robey EA, Weinmaster G. Fringe glycosyltransferases differentially modulate Notch1 proteolysis induced by Delta1 and Jagged1. Mol Biol Cell. Feb 2005;16(2):927-942. 32. Moran JL, Shifley ET, Levorse JM, et al. Manic fringe is not required for embryonic development, and fringe family members do not exhibit redundant functions in the axial skeleton, limb, or hindbrain. Dev Dyn. Jul 2009;238(7):1803-1812. 33. Benedito R, Roca C, Sorensen I, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. Jun 12 2009;137(6):1124-1135. 34. Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature. Jul 23 1998;394(6691):377-381. 35. Xu K, Nieuwenhuis E, Cohen BL, et al. Lunatic Fringe-mediated Notch signaling is required for lung alveogenesis. Am J Physiol Lung Cell Mol Physiol. Jan;298(1):L4556. 36. Tan JB, Xu K, Cretegny K, et al. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity. Feb 20 2009;30(2):254-263. 18 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 37. Visan I, Tan JB, Yuan JS, Harper JA, Koch U, Guidos CJ. Regulation of T lymphopoiesis by Notch1 and Lunatic fringe-mediated competition for intrathymic niches. Nat Immunol. Jun 2006;7(6):634-643. 38. Tsukumo S, Hirose K, Maekawa Y, Kishihara K, Yasutomo K. Lunatic fringe controls T cell differentiation through modulating notch signaling. J Immunol. Dec 15 2006;177(12):8365-8371. 39. Koch U, Lacombe TA, Holland D, et al. Subversion of the T/B lineage decision in the thymus by lunatic fringe-mediated inhibition of Notch-1. Immunity. Aug 2001;15(2):225-236. 40. Acar M, Jafar-Nejad H, Takeuchi H, et al. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. Jan 25 2008;132(2):247-258. 41. Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. Apr 2007;134(7):12431251. 42. Wendorff AA, Koch U, Wunderlich FT, et al. Hes1 is a critical but context-dependent mediator of canonical Notch signaling in lymphocyte development and transformation. Immunity. Nov 24 2010;33(5):671-684. 43. Talora C, Campese AF, Bellavia D, et al. Pre-TCR-triggered ERK signallingdependent downregulation of E2A activity in Notch3-induced T-cell lymphoma. EMBO Rep. Nov 2003;4(11):1067-1072. 44. Gonzalez-Garcia S, Garcia-Peydro M, Martin-Gayo E, et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R{alpha} gene expression in early human thymopoiesis and leukemia. J Exp Med. Apr 13 2009;206(4):779-791. 45. Bigas A, Robert-Moreno A, Espinosa L. The Notch pathway in the developing hematopoietic system. Int J Dev Biol. 2010;54(6-7):1175-1188. 46. Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. Mar 2011;138(6):1017-1031. 47. Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. Sep 2005;33(9):1041-1047. 48. Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. Oct 2006;11(4):519-533. 49. Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. Aug 2001;29(8):927-936. 50. Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. Feb 1998;125(4):725-732. 19 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 51. Fehling HJ, Lacaud G, Kubo A, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. Sep 2003;130(17):4217-4227. 52. Dieterlen-Lievre F, Beaupain D, Martin C. Origin of erythropoietic stem cells in avian development: shift from the yolk sac to an intraembryonic site. Ann Immunol (Paris). Nov-Dec 1976;127(6):857-863. 53. Dieterlen-Lievre F, Pardanaud L, Yassine F, Cormier F. Early haemopoietic stem cells in the avian embryo. J Cell Sci Suppl. 1988;10:29-44. 54. Cormier F, Dieterlen-Lievre F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development. Feb 1988;102(2):279-285. 55. Caprioli A, Minko K, Drevon C, Eichmann A, Dieterlen-Lievre F, Jaffredo T. Hemangioblast commitment in the avian allantois: cellular and molecular aspects. Dev Biol. Oct 1 2001;238(1):64-78. 56. Kanatsu M, Nishikawa SI. In vitro analysis of epiblast tissue potency for hematopoietic cell differentiation. Development. Mar 1996;122(3):823-830. 57. Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. Jun 24 1997;94(13):6776-6780. 58. Alvarez-Silva M, Belo-Diabangouaya P, Salaun J, Dieterlen-Lievre F. Mouse placenta is a major hematopoietic organ. Development. Nov 2003;130(22):54375444. 59. Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. Mar 2005;8(3):365-375. 60. Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. Mar 2005;8(3):377-387. 61. Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897-906. 62. de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. May 2002;16(5):673-683. 63. Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. Apr 26 2007;446(7139):1056-1061. 64. Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. Feb 12 2009;457(7231):887-891. 20 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 65. Zovein AC, Hofmann JJ, Lynch M, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. Dec 4 2008;3(6):625-636. 66. Lawson ND, Scheer N, Pham VN, et al. Notch signaling is required for arterialvenous differentiation during embryonic vascular development. Development. Oct 2001;128(19):3675-3683. 67. Duarte A, Hirashima M, Benedito R, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. Oct 15 2004;18(20):2474-2478. 68. Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. Oct 15 2004;18(20):2469-2473. 69. Kumano K, Chiba S, Kunisato A, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. May 2003;18(5):699-711. 70. Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. Mar 2005;132(5):1117-1126. 71. Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. Oct 1 2005;19(19):2331-2342. 72. You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. May 5 2005;435(7038):98-104. 73. Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFbeta coreceptor. Dev Biol. Sep 1 2003;261(1):235-250. 74. Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat Genet. Nov 2000;26(3):328-331. 75. Xue Y, Gao X, Lindsell CE, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8(5):723-730. 76. Robert-Moreno A, Guiu J, Ruiz-Herguido C, et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. Embo J. Jul 9 2008;27(13):1886-1895. 77. Robert-Moreno A, Espinosa L, Sanchez MJ, de la Pompa JL, Bigas A. The notch pathway positively regulates programmed cell death during erythroid differentiation. Leukemia. Jul 2007;21(7):1496-1503. 78. Hadland BK, Huppert SS, Kanungo J, et al. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. Nov 15 2004;104(10):3097-3105. 21 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 79. Bertrand JY, Cisson JL, Stachura DL, Traver D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood. Apr 8 2010;115(14):2777-2783. 80. Harman BC, Jenkinson WE, Parnell SM, Rossi SW, Jenkinson EJ, Anderson G. T/B lineage choice occurs prior to intrathymic Notch signaling. Blood. Aug 1 2005;106(3):886-892. 81. Milner LA, Kopan R, Martin DI, Bernstein ID. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83(8):2057-2062. 82. Milner LA, Bigas A, Kopan R, Brashem-Stein C, Bernstein ID, Martin DI. Inhibition of granulocytic differentiation by mNotch1. Proc.Natl.Acad.Sci.U.S.A. 1996;93:1301413019. 83. Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol.Cell Biol. 1998;18(4):2324-2333. 84. Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. Oct 23 2003;425(6960):841-846. 85. Maillard I, Koch U, Dumortier A, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. Apr 10 2008;2(4):356-366. 86. Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10(5):547-558. 87. Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID. Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest. Mar 1 2011;121(3):1207-1216. 88. Besseyrias V, Fiorini E, Strobl LJ, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med. Feb 19 2007;204(2):331-343. 89. Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. Oct 2008;9(10):1091-1094. 90. Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. Sep 1999;11(3):299-308. 91. Aifantis I, Raetz E, Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol. May 2008;8(5):380-390. 92. Aster JC. Deregulated NOTCH signaling in acute T-cell lymphoblastic leukemia/lymphoma: new insights, questions, and opportunities. Int J Hematol. Nov 2005;82(4):295-301. 22 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 93. Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. Jul 7 2011;475(7354):101-105. 94. Tanigaki K, Han H, Yamamoto N, et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. May 2002;3(5):443-450. 95. Saito T, Chiba S, Ichikawa M, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. May 2003;18(5):675-685. 96. Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med. May 2 2005;201(9):1361-1366. 97. Kim YW, Koo BK, Jeong HW, et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood. Dec 1 2008;112(12):4628-4638. 98. Klinakis A, Lobry C, Abdel-Wahab O, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. May 12 2011;473(7346):230-233. 99. Qyang Y, Chambers SM, Wang P, et al. Myeloproliferative disease in mice with reduced presenilin gene dosage: effect of gamma-secretase blockage. Biochemistry. May 11 2004;43(18):5352-5359. 100. Yao D, Huang Y, Huang X, et al. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood. May 26 2011;117(21):5652-5662. 101. Mercher T, Cornejo MG, Sears C, et al. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. Sep 11 2008;3(3):314326. 102. Demehri S, Liu Z, Lee J, et al. Notch-deficient skin induces a lethal systemic Blymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. May 27 2008;6(5):e123. 103. Dumortier A, Durham AD, Di Piazza M, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One. 2010;5(2):e9258. 104. Mansson R, Hultquist A, Luc S, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. Apr 2007;26(4):407-419. 105. Katsura Y, Kawamoto H. Stepwise lineage restriction of progenitors in lymphomyelopoiesis. Int Rev Immunol. Feb 2001;20(1):1-20. 23 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 106. Kawamoto H, Katsura Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. May 2009;30(5):193-200. 107. Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. Aug 7 2006;203(8):1867-1873. 108. Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive notch1 signaling. Nat Med. 2000;6(11):1278-1281. 109. Ohishi K, Varnum-Finney B, Bernstein ID. Delta-1 enhances marrow and thymus repopulating ability of human CD34(+)CD38(-) cord blood cells. J Clin Invest. Oct 2002;110(8):1165-1174. 110. Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. Feb 2010;16(2):232-236. 111. Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the selfrenewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. Mar 5 2010;6(3):251-264. 112. Karanu FN, Murdoch B, Gallacher L, et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med. Nov 6 2000;192(9):1365-1372. 113. Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1-induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood. 1999;93(3):838-848. 114. Shimojo H, Ohtsuka T, Kageyama R. Dynamic expression of notch signaling genes in neural stem/progenitor cells. Front Neurosci. 2011;5:78. 115. Yoshiura S, Ohtsuka T, Takenaka Y, Nagahara H, Yoshikawa K, Kageyama R. Ultradian oscillations of Stat, Smad, and Hes1 expression in response to serum. Proc Natl Acad Sci U S A. Jul 3 2007;104(27):11292-11297. 116. Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. Apr 10 2008;58(1):52-64. 117. Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. Nov 2008;11(11):1247-1251. 118. Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. Jul 2002;3(7):517-530. 119. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. Apr 17 2009;137(2):216-233. 24 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 120. Yamamoto N, Tanigaki K, Han H, Hiai H, Honjo T. Notch/RBP-J signaling regulates epidermis/hair fate determination of hair follicular stem cells. Curr Biol. Feb 18 2003;13(4):333-338. 121. Demehri S, Kopan R. Notch signaling in bulge stem cells is not required for selection of hair follicle fate. Development. Mar 2009;136(6):891-896. 122. Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. Nov 2006;133(22):4427-4438. 123. Rangarajan A, Talora C, Okuyama R, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. Jul 2 2001;20(13):3427-3436. 124. Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. Mar 2003;33(3):416-421. 125. Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. Oct 2003;3(10):756-767. 126. Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. Nov 1 2006;20(21):3022-3035. 127. Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. Jul 7 2009;16(1):55-66. 128. Pan Y, Lin MH, Tian X, et al. gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. Nov 2004;7(5):731-743. 129. Weber S, Niessen MT, Prox J, et al. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development. Feb 2011;138(3):495-505. 130. Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. Jan 20 2011;469(7330):415-418. 131. Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. Oct 13 2011;478(7368):255-259. 132. Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. Jan 29 2010;327(5965):542-545. 133. Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. Jun 16 2005;435(7044):964-968. 134. Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. Jan 2000;24(1):36-44. 25 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. 135. Fre S, Hannezo E, Sale S, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One. 2011;6(10):e25785. 136. Pellegrinet L, Rodilla V, Liu Z, et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. Apr 2011;140(4):1230-1240 e1231-1237. 137. Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. Jan 8 2010;327(5962):210-213. 138. Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. Jun 9 2011;474(7350):220-224. 139. Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. Jul 24 2008;454(7203):528-532. 140. Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. Mar 2005;4(3):407-410. 141. Kageyama R, Niwa Y, Shimojo H, Kobayashi T, Ohtsuka T. Ultradian oscillations in Notch signaling regulate dynamic biological events. Curr Top Dev Biol. 2010;92:311-331. FIGURE LEGENDS Figure 1: The Notch signaling pathway Signal-Sending Cell: Functional Notch ligands are ubiquitinated by the E3-ubiquitin ligases mindbomb or neuralized. After ligands interact with the Notch receptor, the ligand and the extracellular part of Notch are endocytosed and ligands may be degraded or recycled. Signal-Receiving Cell: The Notch mRNA is translated as a precursor protein, which is cleaved by a furin-like convertase in the Golgi apparatus to produce a functional heterodimeric receptor. During ER/Golgi transit, Notch is modified by different glycosyltransferases (Rumi/poglut, pofut, fringes). In cells that express fringe, specific sugar moieties (diamonds) are conjugated to confer higher affinity to Delta-type ligands. After ligand binds to the EGF-like repeats of the Notch extracellular domain, an ADAM metalloprotease cleaves Notch at the S2 site, removing most of the extracellular domain. The membrane-tethered intracellular domain is then cleaved by the Presenilin complex at site S3, either in the plasma membrane or after endocytosis, freeing the Notch Intracellular 26 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. domain (ICN). ICN translocates to the nucleus, displaces the corepressor complex, associates with RBP-J and recruits Coactivators such as Mastermind. ICN becomes monoubiquitylated (Ub), targeting the receptor for degradation. Several E3 ubiquitin ligases (Deltex, Nedd4, Su(Dx)/Itch, Cbl) can direct Notch receptor trafficking toward lysosomal degradation or toward recycling. Numb can also promote Notch degradation in daughters of an asymmetrically dividing cell. Figure 2: Pleiotropic Notch functions in the hematopoietic system A) Notch is required during hematopoietic development to generate HSC in the AGM. This process takes place in the aorta endothelium and is dependent of the Jagged1 ligand and the Notch1 receptor. Endothelial-like Pre-HSC or HSC cells are the putative target of Notch activation (light green). B-C) Models of Notch signaling in Hematopoietic differentiation. B) Notch is involved at different steps of differentiation of the hematopoietic lineages. The classical hierarchical model of the hematopoietic tree is here adapted to the concept that a gradient of Notch activation or signaling determines the different types of hematopoietic cells. C) A Hematopoietic potential restriction model107 in which lineage restriction is determined by Notch-doses. HSC progressively lose their capacity to generate different lineages concomitantly to different exposure to Notch-doses. Intensity of the blue color represents a speculative gradient of Notch activity. Dashed lines represent cell potential to become a specific cell type. Solid lines represent progression through normal hematopoietic development. Long-term HSC (LT-HSC), Common myeloid Progenitor (CMP), Common Lymphoid Progenitor (CLP), Lymphoid/Myeloid Potential Progenitor (LMP), Common Megakaryocyte-Erythroid progenitor (CME), Early T-progenitor (ETP), Erythrocyte (Ery), Megakaryocyte (Meg), Macrophage/Granulocyte (M/Gr). Specific Notch activity is indicated (N). 27 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Figure 3: Notch-dependent mechanistic strategies for stem cell maintenance and differentiation. A and B) A)Neuronal differentiation in the developing neuroepithelium is dependent on an asymmetric cell division in which Numb is asymmetrically distributed and inhibits Notch signaling in one of the cells to permit neuronal differentiation. B) In the developing brain, Notch controls the oscillation of downstream genes (hes1 and Ngn2) that allows controlled proliferation and differentiation waves in the midbrain compartment. In the boundary (green boxes), Hes1 protein levels are sustained, Ngn2 levels are low and proliferation and neuron formation is inhibited (based on revision141). C and D) C)Notch promotes cell proliferation in the basal layer of the embryonic epidermis however in adult epidermis D) Notch is inhibiting proliferation and inducing differentiation of the interfollicular basal layer. In the bulge, Notch and Jag1 are regulating epidermal stem cells by promoting the follicular stem cell fate downstream of Wnt/β-catenin. E) Two types of ISC have been identified: Lgr5+ proliferating cells and quiescent +4 cells (Bmi+). Lgr5+ cells are located at the bottom of the intestinal crypts, next to an intestinal secretory-type cell called Paneth cell. These cells are crucial ISC-niche elements, they expresses Notch-ligands Dll1 and Dll4 and are required to maintain ISC (likely through Notch activation). However, differentiation to the secretory lineage including Paneth Cells require Atonal, which is a target of Notch/hes1 repression, suggesting that generation of the ISC-niche is under the control of ISC through Notch. F) Notch signaling induced by Delta1 is required for muscle regeneration upon injury. The balance between Notch and TGFb/smad3 signals determine the levels of CDK inhibitors and the proliferative capacity of muscle progenitors. 28 From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Figure 1. Notch signaling Mind bomb Signal‐sending Cell Ligand recycling Jagged Ligand Activation Neuralized Fng‐modified Notch receptor Delta recycling ADAM(S2) Nicastrin Presenilin γ‐secretase (S3) γ‐secretase Degradation HDAC NCoR KDM5 RBP‐J NOTCH OFF HAT sharp Mam CDK8 RBP‐J NOTCH ON Signal‐receiving Cell From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Figure 2. Notch in the hematopoietic system A EMBRYONIC HEMATOPOIESIS ADULT HEMATOPOIESIS LT-HSC C B LMPP Circulating cells N1-Jag1 Aortic endothelium CMP LMPP ETP ETP T-cell CMP LT-HSC CME Ery B-cell Meg M/Gr mesenchyme Ery Meg M/Gr B-cell M T-cell From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Figure 3. Notch-dependent mechanistic strategies to generate or maintain Stem Cells A NEUROEPITHELIUM Symmetric C Asymmetric N1-Dll1 hes oscillation neuron Numb Astrocyte protein Time protein Low Proliferation Hes1 Ngn2 Time Hes1 Hindbrain β-catenin Jagged1 Notch1 E ADULT INTESTINE Secretory lineages Midbrain High Proliferation Ngn2 Forebrain Notch Notch Epyndemal N1 Radial Glial Oligodendrocyte B DEVELOPING BRAIN D ADULT EPIDERMIS EMBRYONIC EPIDERMIS Atonal ISC (Bmi1+) Paneth cells Hes1 Notch Enterocyte progenitor injury Dll1 Notch Satelite cell Myogenic progenitor N1/N2 CDK inhibitors ISC (Lgr5+) Dll1/4 Proliferating/differentiating compartment F ADULT MUSCLE Stem Cell compartment TGFβ/smad3 Notch myoblast From www.bloodjournal.org by guest on June 14, 2017. For personal use only. Prepublished online February 3, 2012; doi:10.1182/blood-2011-10-355826 Hematopoietic stem cells: to be or Notch to be Anna Bigas and Lluis Espinosa Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Advance online articles have been peer reviewed and accepted for publication but have not yet appeared in the paper journal (edited, typeset versions may be posted when available prior to final publication). Advance online articles are citable and establish publication priority; they are indexed by PubMed from initial publication. Citations to Advance online articles must include digital object identifier (DOIs) and date of initial publication. Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.