* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The process of beta oxidation is named after the carbon atom in the
Proteolysis wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Butyric acid wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemistry wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
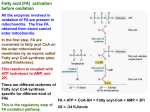
The process of beta oxidation is named after the carbon atom in the beta position of the fatty acyl-CoA which becomes the most oxidized during the cyclic redox reactions that remove C2 units in form of acetyl-CoA from the fatty acyl chain. The beta carbon becomes the new carboxyl end of the shortened (n-2) fatty acyl-CoA. The oxidation steps are strictly analogous to the reaction steps in the citric acid cycle converting succinyl-CoA to oxaloacetate involving an initial oxidation by acyl-CoA dehydrogenase (EC 1.3.99.3; driven by FAD reduction), an hydration by enoyl-CoA hydratase (EC 4.2.1.17), and a second oxidation by hydroxyacyl-CoA dehydrogenase (EC 1.1.1.35 driven by NAD+ reduction). A C2 unit is released by beta-ketothiolase (EC 2.3.1.16) to produce acetyl-CoA and a shortened acyl(n-2)-CoA. The latter is recycled until the acyl chain is shortened to its acetyl-CoA end product and oxidized by the citric acid cycle enzymes. The acyl-CoA dehydrogenase is specific for the length of the acyl chain being oxidized. Three types of the dehydrogenase exist in mitochondria; type I (EC 1.3.99.12; long chain) which oxidizes C12-C18 fatty acids, type II (EC 1.3.99.3) which oxidizes C4-C14 fatty acids, and type III (EC 1.3.99.2; butyryl dehydrogenase) which oxidizes C4 and C6 acyl-CoA substrates. The energy yield per cycle is 5 mols of ATP for each round, 2 mols per FADH2 (goes into complex II) and 3 mols per NADH/H+ (goes into complex I). The energy balance for palmitic acid (sixteen carbon atoms) is: CH3(CH2)14CO-S-CoA + 7H2O +7CoA + 7FAD + 7NAD+ 8CH3-CO-S-CoA + 7FADH2 + 7NADH + 7H+ The completion of the degradation process (coenzyme oxidation) requires the citric acid cycle which yields an additional 96 mols of ATP for all 8 acetyl-CoA units oxidized in the process. The total energy yield of palmitic acid oxidation results in some 130 mols of ATP, 34 units from the beta-oxidation cycle and 96 form the citric acid cycle. Beta oxidation of odd numbered fatty acids yields a (C2) acetyl-CoA and (C3) propionyl-CoA end product during the very last cycle. Propionyl-CoA cannot be further oxidized as such and is converted to methyl-malonyl-CoA by propionyl carboxylase (EC 4.1.1.41). This is an energy consuming step using 1 mol of ATP per mol of propionyl-CoA. The net reaction is: propionyl-CoA + CO2 + ATP = (D/L)-methylmalonyl-CoA + ADP + Pi The methylmalonyl is formed as racemic mixture containing equal amounts of the Dand L-enantiomer. The (D)methylmalonyl-CoA is isomerized to succinyl-CoA by methylmalonyl CoA mutase: (D)-methylmalonyl-CoA = succinyl-CoA Note that this reaction is also used for the degradation of hydrocarbon side chains of the amino acids valine, leucine, and isoleucine; (S)-methylmalonyl is the same as Lmethylmalonyl; from KEGG pathway MAP00280) Similarly, unsaturated fatty acids need special enzymes to provide the beta oxidation intermediate trans-D2-enoyl-CoA, the substrate of enoyl-CoA hydratase. Desaturation can result in two unusual degradation intermediates: the cis-3- and the cis-4-enoyl-CoA. The beta oxidation intermediate, however is a trans-2 isomer. The cis-3-enoyl-CoA intermediate is isomerized to the trans-2 form by isomerase (EC 5.1.2.3). The cis-4-enoyl-CoA form is converted in two steps to cis-3-enoylCoA intermediate. The first step is catalyzed by acyl-CoA dehydrogenase (the first enzyme in the beta oxidation cycle) to form 2,4-dienoyl-CoA which in turn is isomerized by 2,4-dienoyl-CoA reductase to cis-3-enoyl-CoA.