* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electron Transport and oxidative phosphorylation (ATP Synthesis)
Survey
Document related concepts
Mitochondrion wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Phosphorylation wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Transcript
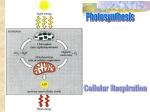
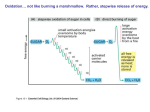
Electron Transport and oxidative phosphorylation (ATP Synthesis) Dr. Howaida Nounou Biochemistry department Sciences college The Metabolic Pathway of Cellular Respiration All of the reactions involved in cellular respiration can be grouped into three main stages Glycolysis – occurs in cytoplasm The Krebs cycle – occurs in matrix of mitochondria Electron transport – occurs across the mitochondrial membrane Mitochondrion Cytosol High-energy electrons carried mainly by NADH High-energy electrons carried by NADH Glycolysis Glucose 2 Pyruvic acid Krebs Cycle Electron Transport A Road Map for Cellular Respiration Redox Reactions Chemical reactions that transfer electrons from one substance to another are called oxidation-reduction reactions REDOX short for oxidation-reduction reactions REDOX FACTS Reduction-oxidation (redox) couple: pair of molecules of which one is reduced and the other is oxidized (e.g., lactate-pyruvate, NADH-NAD+, and FADH2-FAD) each pair constitutes a half reaction the reductant of one pair donates electrons and the oxidant of the other pair accepts the electrons Red1 + Ox2 Ox1 + Red2 REDOX FACTS A:H Reductant B B:H Oxidant + e- Reductant (acceptor) (donor) ² A Oxidant + e- both oxidation and reduction must occur simultaneously Electrons can move through a chain of donors and acceptors In the electron transport chain, electrons flow down a gradient Electrons move from a carrier with low reduction potential (high tendency to donate electrons) toward carriers with higher reduction potential (high tendency to accept electrons) Oxidative process Phosphorylation process O2 e inner membrane H 2O H+ ADP+ Pi H+ outer membrane ATP intermembrane space matrix Figure: Essential features of oxidative phosphorylation Redox reactions of respiratory chain use electrons to reduce oxygen to water Energy generated moves protons from matrix to intermembrane space Inward movement of protons recovers this energy to promote formation of ATP in the matrix. Succinate II NADH I Coenzyme Q III Cytochrome C IV electron flow The components of the RC are arranged in order of increasing redox potential ½ O2 RC exists as four large, multisubunit protein complexes The respiratory electron transport chain complex I is a NADHubiquinone reductase complex II is succinate dehydrogenase (part of the TCA cycle) complex III is the ubiquinone -cytochrome c reductase complex IV is cytochrome oxidase Figure: Complex I of the respiratory chain that links NADH and coenzyme Q. NADH Dehydrogenase accepts 2e-- from NADH and transfers them to ubiquinone (coenzyme Q), an electron carrier Uses two bound cofactors to accomplish this: FMN (Flavin mononucleotide) and 6 iron-sulfur (Fe-S) protein Pumps 4 H+ out to inter-membrane space Complex II: Succinate-CoQ reductase Prosthetic groups: FAD; Fe-S Succinate FAD SDH Fumarate FADH2 CoQ SDH is succinic dehydrogenase an enzyme of the citric acid cycle (associated with membrane) 2 e- transferred from succinate to CoQ 1 mole FADH2 produced Electrons from complex I or II CoQ cyt b/cyt c1 Complex III: cytochrome reductase Prosthetic groups: heme b; heme c1; Fe-S cyt c Figure: Complex III of the respiratory chain linking CoQ and cytochrome C. Is composed of cytochome b, cytochrom C1 and iron sulphur proteins Accepts e- from coenzyme Q and transfers e- to cytochrome c coupled with the transfer of protons from the matrix to the intermembrane space Figure: Complex IV -cytochrome oxidase- reducing oxygen to water Contains cytochromes a/a3 and 2 Cu ions involved in e- transfers Cytochrome oxidase passes electrons from cytochrome c through a series of heme groups and Cu ions to O2, reducing it to H2O (end product) and pumping one proton into the intermembrane space for each e- Coenzymes and cytochromes in the complexes act as e- donors & acceptors Flavin MonoNucleotide (FMN), in Complex I, functions like FAD (Flavin adenine dinucleotide , which is an electron acceptor that helps electron transfer during Krebs Cycle and Electron Transport Chain in cellular respiration). iron-sulfur (Fe-S proteins): Fe-S centers transfer e- in Complexes I, II and III Coenzyme Q (ubiquinone), lipid soluble, floats in the membrane and doesn’t require protein Cytochromes (b, c1, c, a, a3; contain heme): transfer e- in Complexes III and IV, Cytc is the only soluble cytochrome NAD+, FMN, CQ are carriers of e- and hydrogen while cytochromes are carriers of electrons only. Summary of redox complexes of the electron transport chain Complex designation I– NADHNADH-Q reductase Functional groups FMN (flavin monomononucleotide); FeFe-S Function oxidizes NADH to NAD+; transfers electrons to coenzyme Q Poisons Rotenone II – SuccinateSuccinate- FAD; FeFe-S Q reductase oxidizes succinate to fumarate with reduction of FAD to FADH2; electron transfer to CoQ III Cytochrome reductase heme b; heme c1; FeFe-S transfers electrons between coenzyme Q and cytochrome C (C becomes reduced) Antimycin A IV Cytochrome C oxidase heme aa- a3 ; Cu oxidizes cytochrome C; reduces ½O2 to H2O Carbon monoxide Cyanide Overall Reaction…. NADH + H+ + ½ O2 NAD+ + H2O Taking into account the protons pumped out: complex I 4 H+ complex III 4 H+ complex IV 2 H+ The equation for electron transfer is: NADH + 11 H+matr + ½ O2 NAD+ + 10 H+intermem + H2O Oxidative Phosphorylation Definition Process in which ATP is formed as a result of transfer of electrons from NADH or FADH2 by a series of electron carriers The electron transport chain generates no ATP directly. Rather, its function is to break the large free energy drop from food to oxygen into a series of smaller steps that release energy. ATP yield Only 4 of 38 ATP ultimately produced by respiration of glucose are derived from substrate-level phosphorylation (2 from glycolysis and 2 from TCA) The vast majority of the ATP (90%) comes from the energy in the electrons carried by NADH and FADH2 ATP-synthase (complex V), ATPV) present in the inner mitochondrial membrane, actually makes ATP from ADP and Pi. ATP used the energy of an existing proton gradient to power ATP synthesis. This proton gradient develops between the intermembrane space and the matrix. This concentration of H+ is the proton--motive force. proton force 22 The ATP synthase molecules are the only place that will allow H+ to diffuse back to the matrix (exergonic flow of H+). This flow of H+ is used by the enzyme to generate ATP a process called chemiosmosis chemiosmosis.. Chemiosmosis: (osmos = push) is the oxidative phosphorylation that results in ATP production in the inner membrane of mitochondria. Properties of ATP Synthase Multisubunit transmembrane protein Molecular mass = ~450 kDa Functional units F0: water-insoluble transmembrane protein (up to 8 different subunits) F1: water-soluble peripheral membrane protein (5 subunits) ,contains the catalytic site for ATP synthesis Flow of 3 protons through ATP synthase leads to phosphorylation of 1 ADP During respiration, most energy flows from glucose -> NADH -> electron transport chain -> proton proton--motive force -> ATP. One sixsix-carbon glucose molecule is oxidized to 6 CO2 molecules. Some ATP is produced by substrate substrate--level phosphorylation during glycolysis and the Krebs cycle, but most ATP comes from oxidative chain). phosphorylation (through electron transport chain). Each NADH from the Krebs cycle and the conversion of pyruvate contributes enough energy to generate a maximum of 3 ATP. ATP. Each FADH2 from the Krebs cycle can be used to generate about 2ATP ATP.. Energy produced in electron transport chain gives a maximum yield of 34 ATP by oxidative phosphorylation via ATPATP-synthase. These compounds prevent the passage of e- by binding a component of the ETC blocking the oxidation/reduction reaction Inhibitors of ElectronTransport Chain and oxidative phosphorylation Be familiar with the actions of inhibitors in the red boxes Uncouplers: Compounds that increase the permeability of the inner mitochondrial membrane to protons. Protons renters the matrix at sites other than ATP synthase through holes made by these compounds . These compounds have no effect on electron transport chain , but they uncouple oxidative phosphorylation. The energy produced by the transport of electron is released as heat rather than being used to synthesis ATP. Examples: 2,4 dinitrophenol, dinitrocresol, pentachlorophenol, thyroxine, calcium, mchlorocarbonylcyanide phenyl hydrazone (cccp). Adding Up the ATP Cytosol Mitochondrion Glycolysis Glucose 2 Pyruvic acid 2 AcetylCoA Krebs Cycle Electron Transport Maximum per glucose: by direct synthesis by direct synthesis by ATP synthase Protein complex Electron carrier Inner mitochondrial membrane Electron flow Electron transport chain ATP synthase 31 Food Polysaccharides Sugars Glycerol Fats Fatty acids Proteins Amino acids Amino groups Glycolysis AcetylCoA Krebs Cycle Electron Transport 32