* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Overview of metabolism
Metabolic network modelling wikipedia , lookup
Metalloprotein wikipedia , lookup
Cofactor engineering wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
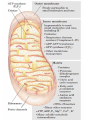
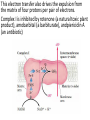
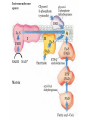
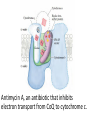
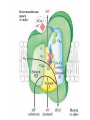
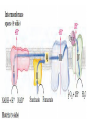
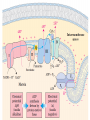
Overview of metabolism http://www.youtube.com/watch?v=kN5MtqAB_Yc Autotrophs • Such as photosynthetic bacteria and vascular plants. • It can use carbon dioxide from the atmosphere as their sole source of carbon, from which they construct all their carbon containing biomolecules. • Autotrophic cells and organisms are relatively self-sufficient. Heterotrophs • It must obtain carbon from their environment in the form of relatively complex organic molecules such as glucose. • Multicellular animals and most microorganisms are heterotrophic. • It must subsist on the products of other organisms. Metabolism Metabolism The sum of all the chemical transformations taking place in a cell or organism occurs through a series of enzyme-catalyzed reactions that constitute metabolic pathways. Catabolism • Is the degradative phase of metabolism in which organic nutrient molecules (carbohydrates, fats, and proteins) are converted into smaller, simpler end products (such as lactic acid, CO2, NH3). • Catabolic pathways release energy, some of which is conserved in the formation of ATP and reduced electron carriers (NADH, NADPH, and FADH2); the rest is lost as heat. Anabolism Also called biosynthesis, small, simple precursors are built up into larger and more complex molecules, including lipids, polysaccharides, proteins, and nucleic acids. Anabolic reactions require an input of energy, generally in the form of the phosphoryl group transfer potential of ATP and the reducing power of NADH, NADPH, and FADH2 Generally • Some metabolic pathways are linear, and some are branched, yielding multiple useful end products from a single precursor or converting several starting materials into a single product. • In general, catabolic pathways are convergent and anabolic pathways divergent. Generally • Some metabolic pathways are linear, and some are branched, yielding multiple useful end products from a single precursor or converting several starting materials into a single product. • In general, catabolic pathways are convergent and anabolic pathways divergent. Generally Some pathways are cyclic: one starting component of the pathway is regenerated in a series of reactions that converts another starting component into a product. All oxidative steps in the degradation of carbohydrates, fats, and amino acids converge at this final stage of cellular respiration, in which the energy of oxidation drives the synthesis of ATP. Generally In eukaryotes, oxidative phosphorylation occurs in mitochondria. Oxidative phosphorylation involves the reduction of O2 to H2O with electrons donated by NADH and FADH2. Biological oxidation NADH:ubiquinone oxidoreductase (Complex I). NADH:ubiquinone oxidoreductase (Complex I). Complex I catalyze the transfer of a hydride ion from NADH to FMN, from which two electrons pass through a series of Fe-S centers to the iron sulfur protein N-2 in the matrix arm of the complex. Electron transfer from N-2 to ubiquinone on the membrane arm forms QH2, which diffuses into the lipid bilayer. This electron transfer also drives the expulsion from the matrix of four protons per pair of electrons. Complex I is inhibited by rotenone (a natural toxic plant product), amobarbital (a barbiturate), andpiericidin A (an antibiotic) Electrons from succinate pass through a flavoprotein and several Fe-S centers (in Complex II) on the way to Q. Glycerol 3- phosphate donates electrons to a flavoprotein (glycerol 3-phosphate dehydrogenase) on the outer face of the inner mitochondrial membrane, from which they pass to Q. Acyl-CoA dehydrogenase (the first enzyme of β- oxidation) transfers electrons to electron transferring flavoprotein (ETF), from which they pass to Q via ETF:ubiquinone oxidoreductase. Oxaloacetate and malonate are competitive inhibitors of succinate dehydrogenase and compete with the substrate for binding at the active site. Carboxin and thenoyltrifluoroacetone inhibit electron transfer from FADH2 to CoQ. Complex III: Ubiquinone to Cytochrome c The next respiratory complex, Complex III, also called cytochrome bc1 complex or ubiquinone: cytochrome c oxidoreductase, couples the transfer of electrons from ubiquinol (QH2) to cytochrome c with the vectorial transport of protons from the matrix to the intermembrane space. Cytochrome c1 and the Rieske iron-sulfur protein project from the P surface and can interact with cytochrome c (not part of the functional complex) in the intermembrane space. Antimycin A, an antibiotic that inhibits electron transport from CoQ to cytochrome c. Path of electrons through Complex IV. • Electron transfer through Complex IV begins when two molecules of reduced cytochrome c each donate an electron to the binuclear center CuA. • From here electrons pass through heme a to the Fe- Cu center (cytochrome a3 and CuB). Oxygen now binds to heme a3 and is reduced to its peroxy derivative by two electrons from the Fe-Cu center. • Delivery of two more electrons from cytochrome c (making four electrons in all) converts the two molecules of water, with consumption of four “substrate” protons from the matrix. At the same time, four more protons are pumped from the matrix by an as yet unknown mechanism. • Cytochrome oxidase is inhibited by cyanide, carbon monoxide, and azide. Chemiosmotic model • electrons from NADH and other oxidizable substrates pass through a chain of carriers arranged asymmetrically in the inner membrane. • Electron flow is accompanied by proton transfer across the membrane, producing both a chemical gradient (ΔpH) and an electrical gradient. • The inner mitochondrial membrane is impermeable to protons; protons can reenter the matrix only through proton-specific channels (Fo). The proton-motive force that drives protons back into the matrix provides the energy for ATP synthesis, catalyzed by the F1 complex associated with Fo. Inhibitors of Respiratory Chain These inhibitors block hydrogen or electron transfer along the chain and are grouped into three groups. At Complex I: They prevent utilization of NADH as a substrate, e.g., barbiturates (sedatives and hypnotics), rotenone (a fish poison and insecticide), piericidin A (an antibiotic) and amytal. At Complex II: They inhibit succinate dehydrogenase, e.g., malonate or inhibit the succinate-Q reductase, e.g., Carboxin. At Complex III: e.g., dimercaprol (used as anti-arsenic) and antimycin A (an antibiotic). They lead to release of H2O2. At Complex IV: e.g., cyanides, azide, carbon monoxide and hydrogen sulfide. At Complex V: They prevent ATP synthesis from ADP + Pi, e.g., oligomycin that also inhibits all the other complexes. Inhibitors of oxidative phosphorylation (uncouplers) They inhibit oxidative phosphorylation by disconnecting phosphorylation (ADP + Pi ATP) from oxidation steps in the respiratory chain. Thus, there will be no ATP production although oxidation steps in the respiratory chain are running. This is due to dissipation of the H+ electrochemical gradient. Thermogenins: They are uncoupler protein channels in the inner mitochondrial membrane of the brown adipose tissue in newborn animals. Calcium Injection: Injection of calcium dissipates H+ gradient used for transport of Ca2+ into mitochondria and liberating free heat and leads to the sensation of increased body temperature. Thyroxine: High concentration of thyroxine dissipates H+ gradient and increasing oxygen consumption. Progesterone: The release of progesterone at the mid-menstrual cycle during ovulation interferes with the oxidative phosphorylation. This increases the female’s body temp. by about 0.5oC that is used as an indicator for time of ovulation. Chlorpromazine: It is an antiemetic drug used to prevent vomiting. Dicumarol, Dinitrophenol, dinitrocresol, pentachloro-phenol : They work + as H channel in mitochondrial membrane dissipating the electrochemical gradient as free heat and no ATP is produced. They were used in treatment of obese persons to reduce their weight. Arsenic compounds: It interferes by being used instead of Pi generating no ATP (see glycolysis). Arsenic is a cumulative toxin, i.e., when it is taken in diet in small amounts it accumulates in the body until it reaches its lethal level leading to death. Oligomycin: This is a fungicidal drug that inhibits ATP synthase.