* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Intro to Nuclear Chemistry
Nuclear fusion–fission hybrid wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Radioactive waste wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear fission wikipedia , lookup
Ionizing radiation wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear fission product wikipedia , lookup
Background radiation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Technetium-99m wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear transmutation wikipedia , lookup
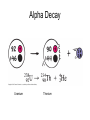
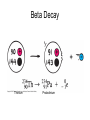
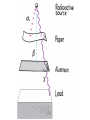
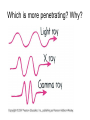
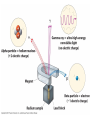
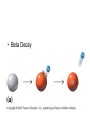
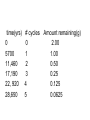
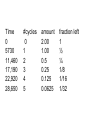
Intro to Nuclear Chemistry Honors Chemistry http://www.chem.orst.edu/graduate/pics/Reactor.jpg How does a nuclear reactor work? http://www.lanl.gov/science/1663/images/reactor.jpg How does a small mass contained in this bomb cause…… • Nuclear Bomb of 1945 known as “fat man” http://www.travisairmuseum.org/assets/images/fatman.jpg …this huge nuclear explosion? http://library.thinkquest.org/06aug/01200/Graphics/705px-Nuclear_fireball.jpg Is there radon in your basement? http://a.abcnews.com/images/Blotter/abc_1radon_ad_070625_ssh.jpg Notation Review Some terms • Nucleons • Radioactivity • Radioisotopes H H http://education.jlab.org/glossary/isotope.html H Isotopes of Carbon • • • • C-11 unstable C-12, C-13 stable C-14, C-15, C-16 unstable Unstable or radioactive isotopes Discovery of Radioactivity • Henri Becquerel 1896 accidentally observed radioactivity of uranium salts • His associates were Marie and Pierre Curie. Marie Curie: born 1867, in Poland as Maria Sklodowska • Lived in France • 1898 discovered the elements polonium and radium. http://www.radiochemistry.org/nuclearmedicine/pioneers/images/mariecurie.jpg Marie Curie a Pioneer of Radioactivity • Winner of 1903 Nobel Prize for Physics with Henri Becquerel and her husband, Pierre Curie. • Winner of the sole 1911 Nobel Prize for Chemistry. Types of Radiation • Alpha a • Beta b • Gamma g Alpha Decay Emission of alpha particles a : • • • • • • Helium nuclei Two protons and two neutrons Charge +2 Mass 4 amu Relatively slow Can be stopped by a sheet of paper, clothing. Alpha Decay Uranium Thorium Alpha Decay http://education.jlab.org/glossary/alphadecay.gif Beta Decay • Beta particles have the same charge and mass as "normal" electrons. • High velocity • Can be stopped by aluminum foil or a block of wood. Beta Decay • Electrons ejected from the nucleus • Atomic number increases by 1 • Mass remains the same Beta Decay Thorium Protactinium Beta Decay Beta decay • A neutron is “transformed” into a proton. • n0 → p+ + b- Gamma Decay • • • • Gamma radiation g Gamma rays are electromagnetic waves. No mass. No charge. – Most Penetrating, can be stopped by 1m thick concrete or a several cm thick sheet of lead. Gamma radiation • 28Ni61 28Ni61 + g • No change in mass or number • Change in nuclear stability More Examples of Radioactive Decay Alpha decay: 230 ? Th 90 230 He4 + Th 90 2 226 Ra 88 Beta decay: 27 ? Mg 12 27 0 + Mg e 12 -1 27 Al 13 Which is more penetrating? Why? Other Particles • • • • • Positron (or beta positive) 11 0 + 11 C e B 6 +1 5 Atomic number decreases p+ → n0 + b+ 14 ? O 8 • 8O14 +1e0 + 7N14 • Electron Capture (K-capture ) • Large nuclei • 37Rb81 + -1e0 → 36Kr81 • p+ → n0 + b+ Additional Reactions • Induced radioactivity • Fission • Fusion STABILITY AND NUCLEAR REACTIONS Nuclear Stability • Depends on a number of factors including: 1. Binding energy 2. Size of the nucleus 3. Neutron to proton ratio Band of Stability Number of Neutrons, (N) Number of Protons (Z) What happens to unstable nuclei? • They undergo decay • The type of decay depends on the reason for the instability Predicting the Decay • Guidelines based on observations Large Nuclei • Nuclides with atomic number > 83 are always unstable or radioactive How do they become more stable? • Losing mass • Usually by alpha decay What type of decay will happen if the nucleus contains too many neutrons? • Beta Decay Example: 14 6 C 14 N 7 + 0 e -1 In N-14 the ratio of neutrons to protons is 1:1 What type of decay occurs if the nucleus contains too many protons? • Positron (beta positive) emission • K-capture (electron capture) Band of Stability Number of Neutrons, (N) Number of Protons (Z) Other interesting facts • Nuclei with even numbers of neutrons and protons tend to be stable • Nuclei with odd numbers of both protons and neutrons are likely to be radioactive • Only four stable isotopes with odd numbers of protons and neutrons are known in nature: • H-1 • Li-6 • B-10 • N-14 HALF-LIFE Radioactive Half-Life (t1/2 ): • The time for half of the radioactive nuclei in a given sample to undergo decay. Graph of Amount of Remaining Nuclei vs Time A=Aoe-lt A Common Radioactive Isotopes Isotope Half-Life Radiation Emitted Carbon-14 5,730 years b, g Radon-222 3.8 days a Uranium-235 7.0 x 108 years a, g Uranium-238 4.46 x 109 years a Radioactive Half-Life How many grams of o 2.00 g sample of C14 will remain in an organic sample after 28,650 years? The half-life C-14 = 5730 years. time(yrs) 0 5700 11,460 17,190 22, 920 28,650 # cycles Amount remaining(g) 0 2.00 1 1.00 2 0.50 3 0.25 4 0.125 5 0.0625 Time 0 5730 11,460 17,190 22,920 28,650 #cycles 0 1 2 3 4 5 amount 2.00 1.00 0.5 0.25 0.125 0.0625 fraction left 1 ½ ¼ 1/8 1/16 1/32 Half-life Equation Ar = Ao x 1/2n n= T/t1/2 T = time elapsed T1/2 = half-life Problem 1 • Iron-59 has a half-life of 45 days. How much of a 4.00g sample will remain after 135 days? • Answer = 0.500g Problem 2 • A sample of 3x107 Radon atoms are trapped in a basement that is sealed. The half-life of Radon is 3.83 days. How many radon atoms are left after 31 days? • answer:1. x105 atoms Problem 3 • A piece of charcoal found at an archeological site originally contained 60.00 g of C-14. Today it contains 3.750 g. How old is it? T1/2 = 5730. years? • Answer = 22,920 years Problem 4 • Strontium-90 is found in nuclear fall-out. How long will it take for a mole of it (90.0g) to decay to a safe level of 0.150 g? T1/2=28.1 yrs • Answer = 259 yrs