* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download emboj2010191-sup
Survey
Document related concepts
Transcript
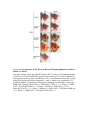
SUPPLEMENTARY INFORMATION Supplementary Figures Figure S1 Malic enzyme is required for malate’s protection against DHEA-induced apoptosis. S2 cells pretreated with either GFP or malic enzyme (Men) dsRNA were treated with 100 µM DHEA ± 5 mM malate in glucose-free medium for 24 h. Lysates from treated cells were then analyzed by caspase assay for effector-like caspase activity. Figure S2 Efficacy of Dronc and Dark dsRNA. Protein levels of Dronc and Dark after RNAi. Lysates from S2 cells treated with Dronc dsRNA were analyzed for endogenous Dronc using anti-Dronc antibodies. The efficacy of Dark RNAi was assessed as previously described (Zimmermann et al, 2002) (Dark1-411, Dark N-terminal truncate mutant containing CARD domain; asterisk, non-specific band). Figure S3 Malate does not interfere with DIAP1 degradation. S2 cells treated with 40 nM DIAP1 dsRNA ± 5 mM malate in serum free Schneider’s medium were collected at indicated time points for immunoblotting using anti-DIAP1 antibodies (Asterisk, non-specific bands as loading control). Figure S4 Men RNAi abrogates malate’s protection. S2 cells pretreated with GFP dsRNA or Men dsRNA pair #2 were treated with 40 μM CHX ± 5 mM malate in standard Schneider’s medium for 12 h. Figure S5 The loss of GFP reflects caspase-mediated decrease in cell viability. Stably transfected S2 cells (see METHODS) were treated with CHX to induce apoptosis. Cells collected at different time points were used to assess the percentage of GFP-positive cells by FACS and then lysed to measure cellular DEVDase activity by caspase assay. Figure S6 Phosphorylation at Dronc S130 does not modulate the interaction between DIAP1 and Dronc. S2 cells were transfected to express FLAG-tagged DIAP1 and HA-tagged catalyticallyinactive Dronc with indicated mutations. Co-immunoprecipitation and expression levels of proteins were performed and examined as described in Fig. 5A. Precipitates on FLAG beads were analyzed with HA antibodies for DIAP1-bound Dronc. Figure S7 PKA and CK1 are unlikely involved in malate’s protection against CHXinduced apoptosis. (A) Neither PKA nor CK1 inhibitors abrogates malate’s protection. S2 cells pretreated with PKA (30 M H-89) (Zhao et al, 2007) or CK1 (8 M IC261) (Mennella et al, 2009) inhibitors were treated with 40 µM CHX ± 5 mM malate in standard Schneider’s medium. Cell density and viability were recorded by pictures. (B) Malate’s protection is not affected by PKA or CK1 dsRNA. S2 cells were pretreated with GFP, PKA or CK1 dsRNA as described in METHODS. Pretreated cells were then treated with 40 µM CHX ± 5 mM malate to examine malate’s protective effect against CHX-induced cell death. Figure S8 Overexpression of WT Dronc or Dronc S130A phosphomutant results in similar eye defects. Two lines strongly expressing either WT Dronc (WTS) or Dronc S130A phosphomutant (S130AS) were crossed with DIAP1-deficient flies (Df(3L)st-f13) or flies constitutively expressing rpr in the eye tissues (GMR-rpr) at 18°C to avoid unnecessary death. Crosses using flies carrying only Dronc transgenes (1× and 2× Dronc) were incubated at 23°C instead to enhance eye phenotype. Expression of transgenes as indicated was driven by GMR-Gal4. Genotype: Driver only (+; GMR-Gal4/+; TM3/+; +), 1× Dronc (+; GMRGal4/+; UAS-Dronc/TM6B; +), 1× Dronc + DIAP1 (+; GMR-Gal4/+; UASDronc/Df(3L)st-f13; +), 1× Dronc + GMR-rpr (+; GMR-Gal4/+; UAS-Dronc/GMR-rpr; +), 2× Dronc (+; GMR-Gal4/+; UAS-Dronc/UAS-Dronc; +). Supplementary Table Table S1. Sequence of oligonucleotide primers used to generate dsRNA templates. Gene FlyBase Annotation Forward primer (5’ to 3’) Reverse primers (5’ to 3’) Dronc Dark drICE DIAP1 Men CG8091 CG6829 CG7788 CG12284 CG10120 CaMKI CaMKII CG1495 CG18069 PKA CK1 CG4379 CG2028 CTCGAGATTGGAATGCCGAAGAGGCAT CGCCCAGCGAAAAATGTATTGATTGATG ATGGACGCCACTAACAATGGAGAATCC ATGGCATCTGTTGTAGCTGATCTTC #1F: GGCTGTTACACGAAGAGAAAC #2F: TACAGTTTGATCCACCAGAAC TACCCTCCGTTTTATGACGAA #1F: ATGCTAACCGTTAATCCAAATAAAC #2F: ATGGCTGCACCAGCAGCCTGTACG ATGATCGTCCAGCACAAGCCC ATGGACAAGATGCGGATATTGAAGG CTGCCCGTGTCGAGATCGCGGTGGGTAAC CCGAATGCTCTCAGCAATAATGCTTAGTCT AACCCGTCCGGCTGGAGCCAACTGCTTGT TCGAAGGTGCGCAGGCG #1R: ATTGTCACCAGTGCCCCAAAG #2R: GGCCACCGACGCGGTTCCTTG CTGCGATGCATTTGTGCTGCC #1R: TTTTTGGGGTATAAAATCGAATGTAGT #2R: GGGCTCCTTTTTCAATACCTCAGG TTCGTAGACGAGTACGCCCAACGC GCGGAACTTCTTGGCCAGACC Table S2. Drosophila stocks from the Bloomington Drosophila Stock center (BDRC) and the Vienna Drosophila RNAi Center (VDRC). Synonyms Source / ID UAS-MEN RNAi hairpins UAS-G6PDH RNAi hairpins GMR-rpr Df(3L)st-f13 VDRC #104016 VDRC #101507 BDRC #5773 BDRC #2993 Supplementary References Mennella V, Tan DY, Buster DW, Asenjo AB, Rath U, Ma A, Sosa HJ, Sharp DJ (2009) Motor domain phosphorylation and regulation of the Drosophila kinesin 13, KLP10A. J Cell Biol 186: 481-490 Zhao Y, Tong C, Jiang J (2007) Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450: 252-258 Zimmermann KC, Ricci JE, Droin NM, Green DR (2002) The role of ARK in stressinduced apoptosis in Drosophila cells. J Cell Biol 156: 1077-1087