* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 11-Electrophoretic method for the separation of LDH
Enzyme inhibitor wikipedia , lookup
Community fingerprinting wikipedia , lookup
Metalloprotein wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Western blot wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
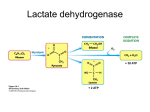
Electrophoretic method for the separation of LDH Isoenzymes On agarose gel The final reaction of anaerobic (without oxygen) glycolysis is the conversion of pyruvate to lactic acid and this reaction is catalyzed by the enzyme lactate dehydrogenase (LDH). In skeletal muscle, where oxygen deprivation is common during exercise, the reaction is efficient and large amounts of lactate can be formed. In tissues that preferentially oxidize glucose aerobically to CO2 and water, such as cardiac muscle, the reaction is not efficient and pyruvate is preferentially converted to acetyl CoA which enters the citric acid cycle. In order to understand the differences in efficiency of this reaction in skeletal and heart muscle, it is necessary to explore the structure of the LDH enzyme in different tissues of the body. Isoenzymes are different molecular forms of the same enzyme, and five major LDH isoenzymes are found in different vertebrate tissues. There are two types of polypeptide chains in LDH called M (for skeletal muscle) and H (for heart muscle) which can be combined into the LDH tetramer in 5 different ways. Each different combination of subunits represents a distinct LDH isoenzyme. Because the H polypeptide has more acidic amino acid residues than the M polypeptide, the electrophoretic mobilities of the LDH isoenzymes are: LDH 1 > LDH 2 > LDH 3 > LDH 4 > LDH 5. LDH isoenzyme 1 is the predominant form of the enzyme in cardiac muscle. The reverse is true in skeletal muscle where there is more M than H polypeptide produced, and hence, more of the isoenzyme 5 forms of the enzyme. The efficiency of the conversion of pyruvate to lactate increases with the number of M chains. Therefore, the high concentration of LDH 5 (4 M subunits) in skeletal muscle rapidly converts pyruvate to lactate while the high concentration of LDH 1 (4 H subunits) in heart tissue favors conversion of pyruvate to acetyl CoA which enters the citric acid cycle. In a myocardial infarction, enhanced enzyme level is proportional to the damage of the heart muscle tissue and in serious cases its elevation could even be three fold. The elevation of serum LDH activity might represent other diseases (e.g. anemia, tumors, liver diseases) as well, so it is important to know from what tissue the LDH was released into the bloodstream. Tissue specific differences in LDH isoenzymes can be readily detected by the localization of LDH activity in an agarose gel after electrophoresis by an activity staining process where the product of the enzymic reaction is a water insoluble stain precipitating in the gel where the LDH proteins are located. Each of the LDH isozymes can catalyze the following reaction: LDH Lactate + NAD+ Pyruvate + NADH + H+ In order to detect the LDH isozyme in an agarose gel after electrophoresis, the above enzymatic reaction is coupled to a color producing reaction: 1) Lactate + NAD+ Pyruvate + NADH + H+ 2) NADH + PMS NAD+ + PMS-H 3) PMS-H + TNBT PMS + TNBT-Formazan. (TNBT- Tetranitroblue tetrazolium) (PMS - Phenazine methosulfate) The highly colored TNBT-Formazan product localizes in the electrophoretic zones of LDH activity and the amount of brown color formed is quantitatively related to the level of LDH isoenzyme present. PROTOCOL Prepare a 1.2% agarose gel in 50 mM Tris–HCl buffer pH 8.2. Load 20 µl from serum specimens into different slots gel. Use bromophenol blue as a tracking dye Carefully cover the samples with tank buffer. Pour tank buffer into the reservoires and connect the electric cables. Electrophorese at 170 V until the bromophenol blue has migrated to within 1 mm of the positive electrode end of the gel. Detection of LDH Isoenzymes Turn off electricity, and place the gel into the developing chamber which already contains the developer solution ( H2O 18.4 ml, 1 M Tris 4 ml, tetrazolium-blue 12 ml, phenazine-methosulphate 4 ml, Na-lactate 4 ml and NAD 1.3 ml). Incubate at 37 oC to develop color reaction for 30 minutes. Data Analysis Rinse the gels with water and examine them on a light box. Locate the bands containing LDH isozymes. The amount of brown color is roughly proportional to the amount of an LDH isoenzyme present in a band. The gels can be stored in standard destain solution containing 10% methanol and 5% acetic acid.