* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chem*3560 Lecture 21: Fatty acid synthase
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Peptide synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Butyric acid wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Catalytic triad wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
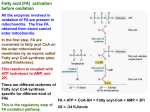
Chem*3560 Lecture 21: Fatty acid synthase Fatty acids are made by a multienzyme complex called fatty acid synthase, which contains seven distinct catalytic centres. Fatty acid synthase is arranged around a central acyl carrier protein, which contains a protein bound pantetheine chain similar to the long chain of Coenzyme A. The pantetheine chain is bonded to a serine side chain of acyl carrier protein (ACP). Pantetheine acts as a long "arm" that fixes the growing acyl chain in the fatty acid synthase, but gives it sufficient mobility in the enzyme to visit the different catalytic sites (Lehninger p.772-776). The catalytic cycle starts with ketoacylACP synthase (KS) which condenses malonyl ACP to the growing acyl chain (R is a simple saturated hydrocarbon chain). The condensation displaces the extra carboxylate from the malonyl-ACP, so that decarboxylation provides driving force for the reaction (Lehninger Fig. 21-2). Next, the ketone group is reduced to a hydroxyl group by ketoacyl ACP reductase (KR), which requires NADPH as H donor. H2 O is eliminated by hydroxyacyl ACP dehydratase (HD) to give the enoyl-ACP. A second reduction step also uses NADPH and is catalyzed by enoyl ACP reductase (ER), yielding a saturated acyl chain that is two C atoms longer than the original R. These four reactions effectively reverse the β-oxidation process. Fatty acid synthase however requires two additional reactions in order to keep the growing chain on the central ACP. The catalytic site of ketoacyl ACP synthase includes a Cys side chain which contributes an auxilary -SH site for the saturated acyl chain. This is indicated in the reaction scheme as R-CO-S-Enz or HS-Enz. The enzyme acyl transferase (AT) plays a dual role: If ACP is currently unoccupied, acyl transferase accepts acetyl CoA as a substrate, and transfers the acetyl group to the HS-Enz position (left). This acts to start a new chain. If ACP is occupied by saturated acyl-S-ACP (the product of enoyl ACP reductase), acyl transferase transfers the saturated acyl from ACP to the auxilary HS-Enz site (right). This allows for another cycle of elongation of an existing chain, and makes HS-ACP available for the next reaction. The enzyme malonyl CoA-ACP transferase (MT) accepts malonyl CoA as substrate, and transfers the malonyl group to HS-ACP to prepare for the next ketoacyl ACP synthase reaction. The ketoacyl ACP synthase then transfers the acyl group from acyl-S-Enz onto the malonyl CH2 , and the resulting ketoacyl product remains bonded to ACP. This means that the growing acyl chain can go through repeated elongation cycles without ever leaving the fatty acid synthase. Fatty acid synthase repeats the reaction cycle seven times The first cycle starts with acetyl CoA (2 C atoms), which is converted to acetyl-S-Enz in the KS catalytic site, and then elongated by reaction with malonyl-S-ACP. Since the malonyl loses its extra carboxylate group, this reaction adds 2 more C atoms to the growing chain. The elongation cycle repeats for a total of 7 cycles, to give the C16 product palmitoyl-S-ACP. Chain length is monitored by the seventh catalytic site in fatty acid synthase, thioesterase (TE). When the acyl chain length reaches 16 C atoms, thioesterase hydrolyses the acyl-S-ACP bond to release free palmitate, C15 H31 CO2 – . palmitoyl-S-ACP + H2 O → palmitate + H+ + HS-ACP Organization of fatty acid synthase Fatty acid synthase consists of the seven catalytic centres arranged around a central ACP. In bacteria such as E.coli, ACP is a small protein of mass 8.8 kDa. Six additional catalytic subunits are arranged around the central ACP (Lehninger p. 777 and Figs. 21-5 to 21-7; however these figures should be regarded as schematic rather than realistic). The pantetheine arm is anchored into ACP, but is long enough to allow covalently bound substrate and intermediates reach all seven catalytic sites. After the acetyl group is first attached to the KS HS-Enz site, the intermediates remain covalently bonded to the enzyme unitl palmitate is finally released. This confines the intermediates to remain within close range of their target catalytic sites and eliminates the inefficiency of random diffusion of intermediates into and out of the surrounding solution. Mammalian fatty acid synthase is a dimer of two polypeptides of 240 kDa each. Each polypeptide contains eight domains that represent the seven catalytic centres plus an integral ACP domain. The pantetheine arm allows intermediates to reach KR, ER and HD sites on the same polypeptide, but KS, AT and MT sites on the opposite polypeptide are closer than those on the same polypeptide. Overall cost of fatty acid synthesis The overall fatty acid synthase equation for seven cycles acetyl-CoA + 7 malonyl-CoA + 14 NADPH → palmitate + 7 CO2 + 8 HSCoA + 14 NADP+ + 14 H+ + 6 H2 O Overall cost of synthesis in bacteria includes 7 ATP as the energy cost of making 7 malonyl-CoA: acetyl CoA carboxylase 7 acetyl-CoA + 7 ATP + 7 CO2 + 7 H2 O → 7 malonyl-CoA + 7 H+ + 7 ADP + 7 Pi Overall cost of synthesis in mammalian cells includes an additional 8 ATP required for citrate lyase citrate lyase 8 citrate + 8 ATP + 8 HSCoA → 8 acetyl-CoA + 8 oxaloacetate + 8 ADP + 8 Pi Sources of NADPH There are three common sources of NADPH (other than in higher plants, where NADPH is produced directly by photosynthesis). The pentose phosphate cycle is a major source of NADPH. Depending on organism and tissue, two other common sources of NADPH may be used: malate 2– malate dehydrogenase (decarboxylating) or malic enzyme + NADP+ → pyruvate – + CO2 + NADPH The enzyme makes oxaloacetate as a bound intermediate, and then decarboxylates it to release pyruvate as a product. The decarboxylation provides the driving force needed to produce excess NADPH. Enz:[oxaloacetate] → pyruvate + CO2 Many organisms also contain an NADP + dependent isozyme of isocitrate dehydrogenase. isocitrate dehydrogenase (NADP + dependent) isocitrate + NADP+ → α -ketoglutarate + CO2 + NADPH Unlike the NAD+ dependent isozyme, this isozyme does not require ADP for activation, so stays active when low levels of ADP cause the normal TCA cycle isocitrate dehydrogenase to lose activity. isocitrate dehydrogenase isocitrate + NAD + –\\→ → α -ketoglutarate + CO2 + NADH no activity at low [ADP]