UV SURFACE ENVIRONMENT OF EARTH
... star receives many times the biologically effective radiation as around the early Sun and thousands of times the modern Earth-Sun levels. A pre-biotic Earth orbiting GJ 581 (M3.5V) receives hundreds times less biologically effective radiation, a few times modern Earth-Sun levels. The UV fluxes calcu ...
... star receives many times the biologically effective radiation as around the early Sun and thousands of times the modern Earth-Sun levels. A pre-biotic Earth orbiting GJ 581 (M3.5V) receives hundreds times less biologically effective radiation, a few times modern Earth-Sun levels. The UV fluxes calcu ...
A New Physical Model for the Atomic Mass
... which yields excellent results. Figure 1 shows the relative errors of the fitted atomic masses. ...
... which yields excellent results. Figure 1 shows the relative errors of the fitted atomic masses. ...
Radiative Processes in Astrophysics
... For a simple model of randomly placed absorbing spheres with a cross-section & and number density n the mean covering factor for objects in a tube of area A and length ds is dA/A = n&dV/A = n&dl so the attenuation of intensity: ...
... For a simple model of randomly placed absorbing spheres with a cross-section & and number density n the mean covering factor for objects in a tube of area A and length ds is dA/A = n&dV/A = n&dl so the attenuation of intensity: ...
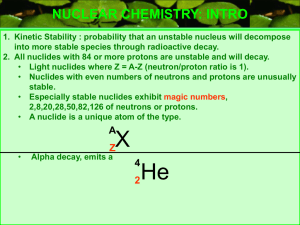
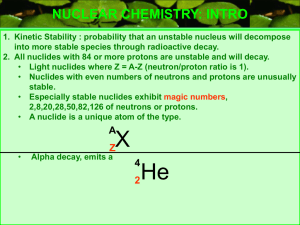
The Band of Stability
... Introduction: Radioactivity is the spontaneous emission of radiation by nuclei. Radioactive decay changes the nature and identity of an atom’s nucleus. This occurs for a specific reason. Elements from hydrogen to lead (atomic numbers 1-82) have stable isotopes in which the tendency of protons to rep ...
... Introduction: Radioactivity is the spontaneous emission of radiation by nuclei. Radioactive decay changes the nature and identity of an atom’s nucleus. This occurs for a specific reason. Elements from hydrogen to lead (atomic numbers 1-82) have stable isotopes in which the tendency of protons to rep ...
The Atom
... ** Also notice there is no change in mass. 5. Nuclide that decay by beta emission have too many neutrons in the nucleus for the number of protons present. ...
... ** Also notice there is no change in mass. 5. Nuclide that decay by beta emission have too many neutrons in the nucleus for the number of protons present. ...
Ernest Rutherford Essay Research Paper Rutherford was
... main components of radiation and named them alpha, beta, and gammy rays. Alpha particles are actually the nuclei of helium atoms. Each alpha particle is made up of two protons and two neutrons, with a charge of 2+ and a mass of 4 atomic mass units. On the average, their speed is about 1/10 the speed ...
... main components of radiation and named them alpha, beta, and gammy rays. Alpha particles are actually the nuclei of helium atoms. Each alpha particle is made up of two protons and two neutrons, with a charge of 2+ and a mass of 4 atomic mass units. On the average, their speed is about 1/10 the speed ...
Radioactivity - Miami Beach Senior High School
... will decrease by 4, and it becomes Thorium, Th-234. U-238 has an atomic number of 92, Th-234 will have an atomic number of ...
... will decrease by 4, and it becomes Thorium, Th-234. U-238 has an atomic number of 92, Th-234 will have an atomic number of ...
Background radiation

Background radiation is the ubiquitous ionizing radiation that people on the planet Earth are exposed to, including natural and artificial sources.Both natural and artificial background radiation varies depending on location and altitude.