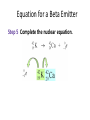* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download mass numbers
Fallout shelter wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear fission product wikipedia , lookup
Ionizing radiation wikipedia , lookup
Background radiation wikipedia , lookup
Radioactive decay wikipedia , lookup
Valley of stability wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear drip line wikipedia , lookup
Tuesday, September 25th Today: •Review •Quiz •Nuclear Chemistry, Chapter 4 •Group Assignment •Test Review Frequency : CD Review Chapter 3 Isotopes Isotopes are atoms of the same element that have different mass numbers. have the same number of protons but different numbers of neutrons. can be distinguished by atomic symbols. Energy Levels and Sublevels Sublevels and Orbitals Each sublevel consists of a specific number of orbitals. An s sublevel contains one s orbital. A p sublevel contains three p orbitals. A d sublevel contains five d orbitals. An f sublevel contains seven f orbitals. Electron Configuration An electron configuration lists the filled and partially filled energy levels in order of increasing energy. lists the sublevels filling with electrons in order of increasing energy. uses superscripts to show the number of electrons in each sublevel. for neon is as follows: number of electrons = 10 1s22s22p6 Abbreviated Configurations In an abbreviated configuration, the symbol of the noble gas is in brackets, representing completed sublevels. the remaining electrons are listed in order of their sublevels. Example: Chlorine has the following configuration: 1s22s22p63s23p5 [Ne] The abbreviated configuration for chlorine is [Ne]3s23p5. Valence Electrons The valence electrons determine the chemical properties of the elements. are the electrons in the outermost, highest energy level. are related to the group number of the element. Example: Phosphorus has 5 valence electrons. 5 valence electrons P Group 5A(15) 1s22s22p63s23p3 Writing Electron-Dot Symbols The electron-dot symbols for Groups 1A (1) to 4A (14) use single dots: Na Mg Al C Groups 5A (15) to 7A (17) use pairs and single dots: P O Cl Atomic Radius Ionization Energy Ionization energy is the energy it takes to remove a valence electron from an atom in the gaseous state. Na(g) + Energy (ionization) Na+(g) + e– decreases down a group, increasing across the periodic table from left to right. Clickers! Frequency: CD How many orbitals are in the d sublevel? a) 1 c) 5 b) 3 d) 7 Which of the following is an alkaline earth metal? a) sodium c) magnesium © 2013 Pearson Education, Inc. b) aluminum d) silicon There are four isotopes of sulfur: 32S, 33S, 34S, and 35S. Based upon the atomic mass listed in the periodic table, 32.07, which isotope is the most abundant? a) 32S c) 34S © 2013 Pearson Education, Inc. b) 33S d) 35S The electron configuration for chlorine, Cl, is ________. a) 1s22s22p63s23p5 b) 1s22s22p63s23p64s1 c) 1s22s22p63s23p6 d) 1s22s22p63s13p5 © 2013 Pearson Education, Inc. What is the correct Electron Dot Symbol for Carbon? a) C b) C c) C d) C Which is the largest element? a) Te c) P b) O d) As Which element has the smallest ionization energy? a) Li c) Ca b) Na d) B Quiz 4 Chapter 4 - Nuclear Chemistry Radioactive Isotopes A radioactive isotope has an unstable nucleus and usually has an atomic number above 20. emits radiation to become more stable. can be one or more of the isotopes of an element. is identified by writing the mass number after the element symbol, such as iodine-131 or I-131. has the following symbol: © 2013 Pearson Education, Inc. Chapter 4, Section 1 22 Some Stable and Radioactive Isotopes © 2013 Pearson Education, Inc. Chapter 4, Section 1 23 Types of Radiation Radiation is the energy emitted by an unstable atom in the process of becoming more stable. It takes the form of alpha particles, which are identical to a helium nucleus, beta particles, which are high-energy electrons with a charge of 1− and a mass number of 0, © 2013 Pearson Education, Inc. Chapter 4, Section 1 24 Types of Radiation (continued) positron, similar to a beta particle with a charge of 1+ and mass number of 0, and gamma rays, which are high-energy radiation often emitted with other types of radiation. They are written with a mass and atomic number of 0. © 2013 Pearson Education, Inc. Chapter 4, Section 1 25 Summary, Types of Radiation © 2013 Pearson Education, Inc. Chapter 4, Section 1 26 Biological Effects of Radiation When radiation strikes molecules, electrons may be knocked away, forming unstable ions. If this ionizing radiation passes through the human body, it may interact with water molecules, removing an electron, producing H2O+. H2O+ can cause undesirable chemical reactions damaging cells. © 2013 Pearson Education, Inc. Chapter 4, Section 1 27 Radiation Protection Radiation protection requires paper and clothing for alpha particles, a lab coat or gloves for beta particles, a lead shield or a thick concrete wall for gamma rays, limiting the amount of time spent near a radioactive source, or increasing the distance from the source. © 2013 Pearson Education, Inc. Chapter 4, Section 1 28 Alpha Decay When a radioactive nucleus emits an alpha particle, a new nucleus forms that has a mass number 4 less than that of the initial nucleus, and an atomic number that has decreased by 2 from that of the initial nucleus. Completing Nuclear Equations In a completed nuclear equation, the sum of the mass numbers of the unstable isotope and the products are equal, and the sum of the atomic numbers of the unstable isotope and the products are equal. Sum of Mass Numbers Sum of Atomic Numbers Guide to Completing a Nuclear Equation Equation for Alpha Decay Write an equation for the alpha decay of 222Rn. Step 1 Write the incomplete equation. 222 86 Rn ? + 4 2 He Step 2 Determine the missing mass number. Step 3 Determine the missing atomic number. Step 4 Determine the symbol of the new nucleus. Equation for Alpha Decay Write an equation for the alpha decay of 222Rn. Step 5 Complete the nuclear equation. Beta Decay A beta particle is an electron emitted from the nucleus. forms when a neutron in the nucleus breaks down into a proton and an electron. Equation for a Beta Emitter Step 1 Write the incomplete equation. 42K (potassium-42), a beta emitter. Step 2 Determine the missing mass number. Step 3 Determine the missing atomic number. Step 4 Determine the symbol of the new Equation for a Beta Emitter Step 5 Complete the nuclear equation. Learning Check Write the nuclear equation for the beta decay of Co-60. Solution Write the nuclear equation for the beta decay of Co-60. Step 1 Write the incomplete equation. Step 2 Determine the missing mass number. Step 3 Determine the missing atomic number. Step 4 Determine the symbol of the new nucleus. Solution Write the nuclear equation for the beta decay of Co-60. Step 5 Complete the nuclear equation. beta particle Positron Emission In positron emission, a proton is converted to a neutron and a positron. the mass number of the new nucleus is the same, but the atomic number decreases by 1. Gamma Radiation In gamma radiation, energy is emitted from an unstable nucleus, indicated by m following the mass number technetium-99m, and the mass number and the atomic number of the new nucleus are for the same element. Summary of Types of Radiation © 2013 Pearson Education, Inc. Chapter 4, Section 1 42 Clicker Review © 2013 Pearson Education, Inc. Chapter 4, Section 1 43 1. An alpha particle is the same as a_____. a) b) c) d) helium-5 nucleus helium-4 nucleus helium-3 nucleus proton 2. The nuclear reaction is an example of______. a) b) c) d) gamma radiation positron emission beta decay alpha decay 3. Uranium-238 decays by emission of an alpha particle. The product of this decay is_____. a) b) c) d) 4. 222R decays to 218P through what process? a) b) c) d) Beta decay Alpha decay Positron emission Gamma emission Producing Radioactive Isotopes Radioactive isotopes are produced when a stable nucleus is converted to a radioactive nucleus by bombarding it with a small particle. in a process called transmutation. Producing Radioactive Isotopes When nonradioactive B-10 is bombarded by an alpha particle, the products are radioactive N-13 and a neutron. © 2013 Pearson Education, Inc. Chapter 4, Section 1 49 Learning Check What radioactive isotope is produced when a neutron bombards 59Co and emits an alpha particle? Exposure to Radiation Exposure to radiation occurs from naturally occurring radioisotopes, including potassium40. medical and dental procedures. air travel, radon, and smoking cigarettes. Radiation Sickness Exposure to radiation of less than 25 rem is usually not detected. Whole-body exposure of 1 Sv produces a temporary decrease in the number of white blood cells. Exposure to radiation greater than 1 Sv may cause radiation sickness. Radiation Sickness This amount of radiation to the whole body is called the lethal dose for one-half the population, or the LD50. LD50 varies for different life forms. For people, whole body radiation of 6 Sv or greater would be fatal within a few weeks. Half-life The half-life of a radioisotope is the time for the radiation level to decrease (decay) to one-half of the original value. Decay Curve A decay curve shows the decay of radioactive atoms and the remaining radioactive sample. Half-life Calculations In one half-life, 40 mg of a radioisotope decays to 20 mg. After two half-lives, 10 mg of a radioisotope remain. Initial 40 mg 2 half-lives 1 half-life 20 mg 10 mg Guide to Calculating Half-lives Sample Calculation of Half-lives P-32, a radioisotope used to treat leukemia, has a half-life of 14.3 days. If a sample contains 8.0 mg of P-32, how many milligrams of P-32 remain after 42.9 days? Step 1 State the given and needed quantities. Analyze the Problem. Given Need 8.0 mg of P-32 half-life of 14.3 days 42.9 days elapsed milligrams of P-32 remaining Sample Calculation of Half-lives P-32, a radioisotope used to treat leukemia, has a half-life of 14.3 days. If a sample contains 8.0 mg of P-32, how many milligrams of P-32 remain after 42.9 days? Step 2 Write a plan to calculate the unknown quantity. days half-life milligrams of 32 15 P number of half-lives number of half-lives milligrams remaining 32 15 P Sample Calculation of Half-lives P-32, a radioisotope used to treat leukemia, has a half-life of 14.3 days. If a sample contains 8.0 mg of P-32, how many milligrams of P-32 remain after 42.9 days? Step 3 Write the half-life equality and conversion factors. Sample Calculation of Half-lives P-32, a radioisotope used to treat leukemia, has a halflife of 14.3 days. If a sample contains 8.0 mg of P-32, how many milligrams of P-32 remain after 42.9 days? Step 4 Set up the problem to calculate the needed quantity. Half-Lives of Some Radioisotopes Radioisotopes that are naturally occurring tend to have long half-lives. used in nuclear medicine have short half-lives. Practice If the half-life of a material is 31.0 days, how many days does a 100.0g sample take to be less than 5.0g remaining? © 2013 Pearson Education, Inc. Chapter 4, Section 1 63 Nuclear Fission In nuclear fission, a large nucleus is bombarded with a small particle, the nucleus splits into smaller nuclei and several neutrons, and large amounts of energy are released. Nuclear Fission When a neutron bombards U-235, an unstable nucleus of U-236 forms and undergoes fission (splits), smaller nuclei are produced, such as Kr-91 and Ba-142, and neutrons are released to bombard more 235U. Nuclear Fission Diagram and Equation 1 0 n + 23592 U 236 92 U © 2013 Pearson Education, Inc. 91 36 1 Kr + 142 Ba + 3 56 0 n + energy Chapter 4, Section 1 66 Learning Check Supply the missing atomic symbol to complete the equation for the following nuclear fission reaction. 1 0 n + 235 92 U 137 52 Te + ? + 210 n + energy Chain Reaction A chain reaction occurs when a critical mass of uranium undergoes fission, releasing a large amount of heat and energy that produces an atomic explosion. Nuclear Power Plants In nuclear power plants, fission is used to produce energy. control rods in the reactor absorb neutrons to slow and control the chain reactions of fission. Nuclear Fusion Nuclear fusion occurs at extremely high temperatures (100,000,000 C). combines small nuclei into larger nuclei. releases large amounts of energy. occurs continuously in the sun and stars. Learning Check Indicate if each of the following describes nuclear fission or fusion, or both. A. A nucleus splits. B. Large amounts of energy are released. C. Small nuclei form larger nuclei. D. Hydrogen nuclei react. E. Several neutrons are released. Group Assignment #4 Next Week • Exam 1, Covering Chapters 1-4 • No Lab