* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download NUCLEAR CHEMISTRY: INTRO
Nuclear and radiation accidents and incidents wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear fission wikipedia , lookup
Background radiation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Technetium-99m wikipedia , lookup
Ionizing radiation wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Radioactive decay wikipedia , lookup
Atomic nucleus wikipedia , lookup
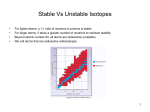
NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with even numbers of neutrons and protons are unusually stable. • Especially stable nuclides exhibit magic numbers, 2,8,20,28,50,82,126 of neutrons or protons. • A nuclide is a unique atom of the type. A X • Z Alpha decay, emits a 4 2 He NUCLEAR CHEMISTRY: STABILITY GRAPH #n 120 110 100 90 80 70 60 50 40 30 20 10 20 p 40 p 60 p 80 p 100 p NUCLEAR CHEMISTRY: BETA DECAY 14 6 1. 2. 3. 4. 5. C 14 N 7 0 + e BETA PARTICLE -1 BETA DECAY THE ATOMIC NUMBER OF THE PRODUCT INCREASES. NUCLIDES ABOVE THE PENNINSULA (ZONE) OF STABILITY DECAY WITH BETA DECAY(SEE GRAPH ON OTHER SLIDE). PENETRATING RADIATION. THE BETA PARTICLE CONES FROM THE DECOMPOSITION OF A NEUTRON TO A PROTON AND BETA PARTICLE. THE BETA PARTICLE IS AN ELECTRON “BORN” IN THE NUCLEUS. BETA DECAY IS SPONTANEOUS, NOTICE ONLY ONE REACTANT. 1 n 0 1 p 1 0 + -1 e NUCLEAR CHEMISTRY: ALPHA DECAY 238 U 92 4 He 2 234 + Th 90 ALPHA PARTICLE 1. 2. 3. 4. 5. 6. 7. ALPHA DECAY THE ATOMIC NUMBER OF THE PRODUCT DECREASES BY 2. THE MASS NUMBER OF THE PRODUCT DECREASES BY 4. ALPHA RADIATION IS NON PENETRATING TO HUMAN SKIN, HOWEVER IT CAN BE INGESTED. COMMON MODE OF DECAY FOR HEAVY RADIOACTIVE NUCLIDES. NEUTRON/PROTON RATIO INCREASES. THE ALPHA PARTICLES ARE POSITIVE, THESE HIGH ENERGY He ATOMS HAVE LOST THE ELECTRONS, ARE REPELLED BY POSITIVE ELECTRODES AND ARE AFFECTED BY MAGNETIC FIELDS. ALPHA DECAY IS SPONTANEOUS. NUCLEAR CHEMISTRY: POSITRON EMISSION 22 11 Na 0 e +1 22 + Ne 10 POSITRON 1. 2. 3. 4. POSITRON EMISSION DECAY MODE FOR NUCLIDES BELOW ZONE OF STABILITY. CHANGES A PROTON TO A NEUTRON. PRODUCT HAS A HIGHER NEUTRON TO PROTON RATIO. THE POSITRON IS THE ANTIPARTICLE TO AN ELECTRON THE REACTION OF A POSITRON WITH A BETA PARTICLE PRODUCES GAMMA RADIATION . 0 e +1 0 + e -1 0 GAMMA 0 NUCLEAR CHEMISTRY: ELECTRON CAPTURE 201 80 Hg 0 + e -1 201 Au 79 + 0 GAMMA 0 INNER ORBITAL SHELL ELECTRON ELECTRON CAPTURE 1. AN INNER SHELL ELECTRON IS CAPTURED BY THE NUCLEUS. FISSION FISSION IS WHERE A LARGE NUCLEOUS IS BOMBARDED BY A SMALL PARTICLE AND THE LARGER NUCLEUS BREAKES DOWN INTO MORE STABLE SMALLER NUCLEII. HERE THE UNSTABLE LARGE NUCLEUS IS URANIUM-235 AND THE BOMBARDMENT PARTICLE IS A NEUTRON. ENOURMOUS QUANTITIES OF ENERGY IS RELEASED NOTICE YOU GET MORE BOMBARDMENT NEUTRONS THAN YOU PUT IN