Chapter 2, section 4 Formation of Elements
... of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. ...
... of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. ...
Isotopes of an atom have the same number of protons, but a different
... ● Gamma rays are waves, not particles. ● This means that they have no mass and no charge. ● In Gamma decay: atomic number unchanged, atomic mass unchanged. ● Gamma rays have a high penetrating power - it takes a thick sheet of metal such as lead to reduce them. ● Gamma rays do not directly ionize ot ...
... ● Gamma rays are waves, not particles. ● This means that they have no mass and no charge. ● In Gamma decay: atomic number unchanged, atomic mass unchanged. ● Gamma rays have a high penetrating power - it takes a thick sheet of metal such as lead to reduce them. ● Gamma rays do not directly ionize ot ...
Radioactive Decay
... Example: Assuming a half-life of _________, how many years will be needed for the decay of ________ of a given amount of radium-226? Amount remaining = 1/16 = 0.0625 = (½)4 = ____________ Years needed for decay of 15/16 = (1599 years) (__) = ___________ ...
... Example: Assuming a half-life of _________, how many years will be needed for the decay of ________ of a given amount of radium-226? Amount remaining = 1/16 = 0.0625 = (½)4 = ____________ Years needed for decay of 15/16 = (1599 years) (__) = ___________ ...
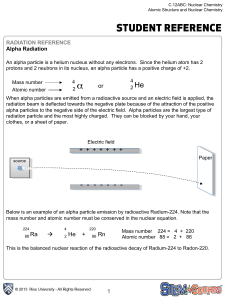
Alpha
... Most radioactive substances have many more particles in their nucleus In some types of atom, the nucleus is unstable, and will decay into a more stable atom. This radioactive decay is completely spontaneous. It's not the same as what happens in a nuclear power station (where neutrons whizz around an ...
... Most radioactive substances have many more particles in their nucleus In some types of atom, the nucleus is unstable, and will decay into a more stable atom. This radioactive decay is completely spontaneous. It's not the same as what happens in a nuclear power station (where neutrons whizz around an ...
Round table on medical applications
... relative biological effectiveness (RBE), as more ionisation events results in a higher probability of causing clustered DNA damage and is thus effective for tumour control. This varies depending on the particle or photon, as seen in figure below, and is due to variations in charge and mass. ...
... relative biological effectiveness (RBE), as more ionisation events results in a higher probability of causing clustered DNA damage and is thus effective for tumour control. This varies depending on the particle or photon, as seen in figure below, and is due to variations in charge and mass. ...
Background radiation

Background radiation is the ubiquitous ionizing radiation that people on the planet Earth are exposed to, including natural and artificial sources.Both natural and artificial background radiation varies depending on location and altitude.