radioactive decay
... Skin can stop alpha radiation Beta radiation – usually only penetrates 1-2cm beneath the skin Alpha, beta and gamma are “ionizing radiation” – they have enough energy to break bonds in molecules which ionizes them, which makes them unstable, and very reactive inside the organism Find out more at Ion ...
... Skin can stop alpha radiation Beta radiation – usually only penetrates 1-2cm beneath the skin Alpha, beta and gamma are “ionizing radiation” – they have enough energy to break bonds in molecules which ionizes them, which makes them unstable, and very reactive inside the organism Find out more at Ion ...
File
... – More penetrating that alpha (pass through paper, but stopped by a thin sheet of metal ...
... – More penetrating that alpha (pass through paper, but stopped by a thin sheet of metal ...
Chapter 25
... Helloween since he was tired of the bad atmosphere in and around the band. Together with Dirk Schlächter and Ralf Scheepers he formed a new band called Gamma Ray. Who will have them on iPod by the end of this unit?? ...
... Helloween since he was tired of the bad atmosphere in and around the band. Together with Dirk Schlächter and Ralf Scheepers he formed a new band called Gamma Ray. Who will have them on iPod by the end of this unit?? ...
Alpha Decay
... rays are often emitted along with alpha or beta particles during radioactive decay. Gamma rays are a radiation hazard for the entire body. They can easily penetrate barriers, such as skin and clothing that can stop alpha and beta particles. Gamma rays have so much penetrating power that several inch ...
... rays are often emitted along with alpha or beta particles during radioactive decay. Gamma rays are a radiation hazard for the entire body. They can easily penetrate barriers, such as skin and clothing that can stop alpha and beta particles. Gamma rays have so much penetrating power that several inch ...
Chapter 25
... 1. What causes a transmutation of the nucleus to occur? 2. How are nuclear decay reaction equations balanced? 3. Do all radionuclides decay at the same rate? ...
... 1. What causes a transmutation of the nucleus to occur? 2. How are nuclear decay reaction equations balanced? 3. Do all radionuclides decay at the same rate? ...
Content Domain III: Chemistry—Atomic Theory and
... unstable, which means that the nucleus emits a particle or radiation with a large amount of energy to help it become more stable. This process is called radioactive decay. When the nucleus in a radioactive material breaks down, it can produce three types of nuclear radiation—alpha, beta, and gamma. ...
... unstable, which means that the nucleus emits a particle or radiation with a large amount of energy to help it become more stable. This process is called radioactive decay. When the nucleus in a radioactive material breaks down, it can produce three types of nuclear radiation—alpha, beta, and gamma. ...
Lecture 3: Interstellar Dust, Radiative Transfer and Thermal Radiation
... •Total energy density uν, per unit frequency at a fixed location (J m-3 Hz-1 or erg cm-3 Hz-1): ...
... •Total energy density uν, per unit frequency at a fixed location (J m-3 Hz-1 or erg cm-3 Hz-1): ...
Chapter 26
... ◦ Beta decay of 14C is used to date organic samples ◦ The ratio of 14C to 12C is used ◦ Ionization-type smoke detectors use a radioactive source to ionize the air in a chamber ◦ A voltage and current are maintained ◦ When smoke enters the chamber, the current is decreased and the alarm sounds ...
... ◦ Beta decay of 14C is used to date organic samples ◦ The ratio of 14C to 12C is used ◦ Ionization-type smoke detectors use a radioactive source to ionize the air in a chamber ◦ A voltage and current are maintained ◦ When smoke enters the chamber, the current is decreased and the alarm sounds ...
Topic 14 - Lloyd Crosby
... b. 1 rem = 1 rad x 1 RBE c. RBE is from Relative Biological Effectiveness. d. The RBE factor depends on how destructive to biological tissues a type of radiation happens to be for the same amount of energy delivered to the tissue e. RBEs for selected radiation (1) X-rays: RBE = 0.7 (2) beta: ...
... b. 1 rem = 1 rad x 1 RBE c. RBE is from Relative Biological Effectiveness. d. The RBE factor depends on how destructive to biological tissues a type of radiation happens to be for the same amount of energy delivered to the tissue e. RBEs for selected radiation (1) X-rays: RBE = 0.7 (2) beta: ...
Nuclear Chemistry - VCC Library
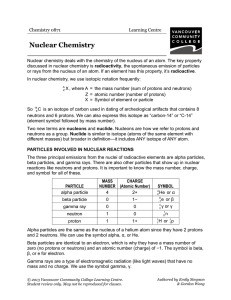
... So 146 C is an isotope of carbon used in dating of archeological artifacts that contains 8 neutrons and 6 protons. We can also express this isotope as “carbon-14” or “C-14” (element symbol followed by mass number). Two new terms are nucleons and nuclide. Nucleons are how we refer to protons and neut ...
... So 146 C is an isotope of carbon used in dating of archeological artifacts that contains 8 neutrons and 6 protons. We can also express this isotope as “carbon-14” or “C-14” (element symbol followed by mass number). Two new terms are nucleons and nuclide. Nucleons are how we refer to protons and neut ...
Background radiation

Background radiation is the ubiquitous ionizing radiation that people on the planet Earth are exposed to, including natural and artificial sources.Both natural and artificial background radiation varies depending on location and altitude.