* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Radioactivity - Miami Beach Senior High School
Isotopic labeling wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Background radiation wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Nuclear fusion wikipedia , lookup
Ionizing radiation wikipedia , lookup
Radioactive decay wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear transmutation wikipedia , lookup
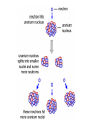
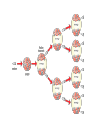
Radioactivity • Elements that emit particles and energy from their nucleus are radioactive. • Some large atoms are unstable and cannot keep their nucleus together. • A radioactive element emits one of these forms of radiation: alpha ( ), beta ( ) and gamma ( ). Alpha particles • An alpha particle is a combination of two protons and two neutrons (the nucleus of a helium atom). • Alpha particles move quickly and have a lot of kinetic energy. They can cause damage to the surface of a material, especially living tissue. They do not penetrate far because they are often trapped by solid materials. • Damage by alpha particles is limited, as they travel through the air and combine with electrons becoming harmless helium. Beta particles • Beta particles are negatively charged particles emitted from the nucleus. • Neutrons can change into protons and electrons when unstable and emit an electron. • They move faster than alpha particles, and so can penetrate light materials, and can get quite deep into the skin, where they can kill cells. • Once stopped they become incorporated into the material. Gamma Rays • Gamma radiation is an extremely energetic form of electromagnetic radiation. • Gamma radiation carries no electric charge and has no mass. • They can penetrate most materials, and can damage molecules in our cells if they penetrate our bodies. • They cannot penetrate unusually dense material like lead. • If an atom changes its number of protons (its atomic number), it becomes a different atom. • If U-238 emits an alpha particle – two protons and two neutrons – its atomic mass will decrease by 4, and it becomes Thorium, Th-234. U-238 has an atomic number of 92, Th-234 will have an atomic number of _________. Emitting beta particles • A neutron can spontaneously transform into a proton and electron in a nucleus with more neutrons than protons. • The electron is emitted form the nucleus. • This is beta radiation. • The element now increases its atomic number by one, as it has an extra proton. • Thorium-234 will become ______-234, after gaining a proton Show how Uranium-238 can become lead by only alpha emissions. Why are elements radioactive? • How is it possible that all positively charged protons in the nucleus remain clumped together? • This question led to the discovery of the strong nuclear force, which acts between all nucleons. • This force is very strong but acts only over very short distances. • The repulsive nuclear force acts over longer distances. • So what happens if the nucleus is large? • A large nucleus is not as stable as a small nucleus. • It emits high energy particles or gamma radiation. • Neutrons act as a nuclear cement. Large nuclei must have more neutrons than protons. • A neutron by itself (not surrounded by protons) is not stable and can spontaneously transform into a proton and an electron. Adding more protons increases the repulsive force. Half-life • The half-life of a radioactive element is the time needed for it to decay to half of the initial amount. • Radium-226 has a half-life of 1620 years. • If you start with 20g, then in 1620 years you will have 10g. • In how many years will there be 5g? In how many will there be 2.5g? Isotopic dating • You can use radioactivity to find the age of rocks and fossils. • For fossils less than 50,000 years old, C-14 will work. • For rocks millions of years old you can use U-238 Carbon-14 • Carbon is a mixture of the two isotopes C-12 and C-14. • C-14 is radioactive and C-12 is stable. • In the air the proportion of C-14 :C-12 is constant. • Plants take in this mixture. Animals get their carbon from plants. • When an animal dies, C-14 starts to decay to N14. So now the proportion of C-14 to C-12 changes. Dating a fossil • If you want to date a fossil you can measure the ratio of C-14 to C-12, compare it to the ratio in the air, and figure out how long ago the creature died. Problems • A sample of radioactive C-14 has decayed to 12.5 % of its original amount. If the halflife of C-14 is 5730 years, how old is the sample? • A fossil has 10.0g of C-14. How long will it take until it contains 0.625g? • Element Z has a half-life of 1 week. Graph the decay of 256g of Z over a 10 week period. Nuclear Fission • Fission is another word for splitting. The process of splitting a nucleus is called nuclear fission. • Uranium isotopes are normally used as the fuel in nuclear reactors because uranium atoms have relatively large nuclei that are easy to split, especially when hit by neutrons. • Fission occurs mainly in the rare isotope U235. Separating U-235 from U-238 is a difficult task. • When a uranium nucleus is hit by a neutron the following happens: • The nucleus splits into two smaller nuclei, which are radioactive. • More neutrons are released. • The additional neutrons released may also hit other uranium nuclei and cause them to split. Even more neutrons are then released, which in turn can split more uranium nuclei. This is called a chain reaction. The chain reaction in nuclear reactors is controlled to stop it going too fast. Nuclear Fusion • Energy is produced as nuclei fuse. • The mass of the fused nucleus is less than the mass of each of the unfused nuclei. • The difference in mass is converted to useful energy. • To fuse the nuclei must be traveling at very high speeds. • This can only occur at extremely high temperatures (thermonuclear fusion) • The hydrogen bomb is a thermonuclear bomb.