The Relationship Between the Nature of the Cell Wall
... ethanol solutions ranging from about 90-100 yo,vlv. Thus under these conditions the CVI in Gram-positiveorganisms remained largely unextractable despite the fact that the CVI complex formed by mixing solutions of the Gram reagents was completely solubilized in such concentrations of aqueous ethanol. ...
... ethanol solutions ranging from about 90-100 yo,vlv. Thus under these conditions the CVI in Gram-positiveorganisms remained largely unextractable despite the fact that the CVI complex formed by mixing solutions of the Gram reagents was completely solubilized in such concentrations of aqueous ethanol. ...
Chapter 9 PP - Jones-Bio
... • Oxygen is the most effective electron acceptor because it is highly electronegative. There is a large difference between the potential energy of NADH and O2 electrons which allows the generation of a large proton-motive force for ATP production. • Cells that do not use oxygen as an electron accept ...
... • Oxygen is the most effective electron acceptor because it is highly electronegative. There is a large difference between the potential energy of NADH and O2 electrons which allows the generation of a large proton-motive force for ATP production. • Cells that do not use oxygen as an electron accept ...
The FAH Fold Meets the Krebs Cycle
... elevated mitochondrial unfolded protein response, different to worms with defects of the electron transport chain. These results clearly demonstrated involvement of fahd-1 in the maintenance of nematode mitochondrial function. Since homologs of FAHD1 are found in all eukaryotic species investigated ...
... elevated mitochondrial unfolded protein response, different to worms with defects of the electron transport chain. These results clearly demonstrated involvement of fahd-1 in the maintenance of nematode mitochondrial function. Since homologs of FAHD1 are found in all eukaryotic species investigated ...
Slide ()
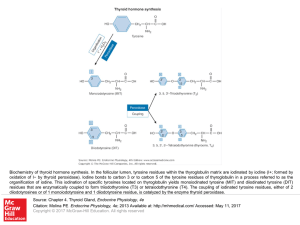
... Biochemistry of thyroid hormone synthesis. In the follicular lumen, tyrosine residues within the thyroglobulin matrix are iodinated by iodine (I+; formed by oxidation of I− by thyroid peroxidase). Iodine bonds to carbon 3 or to carbon 5 of the tyrosine residues of thyroglobulin in a process referred ...
... Biochemistry of thyroid hormone synthesis. In the follicular lumen, tyrosine residues within the thyroglobulin matrix are iodinated by iodine (I+; formed by oxidation of I− by thyroid peroxidase). Iodine bonds to carbon 3 or to carbon 5 of the tyrosine residues of thyroglobulin in a process referred ...
Biology: Concepts and Connections, 6e
... A) The electrons move from carriers that have more affinity for them to carriers that have less affinity for them. B) Molecular oxygen is eventually oxidized by the electrons to form water. C) The electrons release large amounts of energy each time they are transferred from one carrier to another. D ...
... A) The electrons move from carriers that have more affinity for them to carriers that have less affinity for them. B) Molecular oxygen is eventually oxidized by the electrons to form water. C) The electrons release large amounts of energy each time they are transferred from one carrier to another. D ...
03CAM 2011 - AP Bio Take 5
... PHYSICALLY separate carbon fixation from Calvin cycle different cells to fix carbon vs. where Calvin cycle occurs store carbon in 4C compounds different enzyme to capture CO2 (fix carbon) ...
... PHYSICALLY separate carbon fixation from Calvin cycle different cells to fix carbon vs. where Calvin cycle occurs store carbon in 4C compounds different enzyme to capture CO2 (fix carbon) ...
Biology: Concepts and Connections, 6e (Campbell)
... A) they will no longer be able to perform anaerobic respiration. B) high levels of fermentation products will build up in their bodies. C) they will no longer be able to produce adequate amounts of ATP. D) they will no longer be able to absorb water and will become dehydrated. E) they will no longer ...
... A) they will no longer be able to perform anaerobic respiration. B) high levels of fermentation products will build up in their bodies. C) they will no longer be able to produce adequate amounts of ATP. D) they will no longer be able to absorb water and will become dehydrated. E) they will no longer ...
Anaerobic Energy Systems
... If the activity is short duration (less than 10 seconds) and high intensity, we use the ATP–PC system. If the activity is longer than 10 seconds and up to 3 minutes at high intensity, we use the lactic acid system If the activity is long duration and submaximal pace, we use the aerobic system. ...
... If the activity is short duration (less than 10 seconds) and high intensity, we use the ATP–PC system. If the activity is longer than 10 seconds and up to 3 minutes at high intensity, we use the lactic acid system If the activity is long duration and submaximal pace, we use the aerobic system. ...
metabolism and function of carbohydrates
... 8. Where in organism and at what physiologic conditions goes the production of lactate? What is its further destiny? Write down the reaction catalyzed by lactate – dehydrogenase 9. Count up the energetic effect of the anaerobic glycolisys. What is the mechanism of АТP formation? 10. What is the dest ...
... 8. Where in organism and at what physiologic conditions goes the production of lactate? What is its further destiny? Write down the reaction catalyzed by lactate – dehydrogenase 9. Count up the energetic effect of the anaerobic glycolisys. What is the mechanism of АТP formation? 10. What is the dest ...
Cellular Respiration: Harvesting Chemical Energy
... • NADH passes the electrons to the electron transport chain • Unlike an uncontrolled reaction, the electron transport chain passes electrons in a series of steps instead of one explosive reaction • O2 pulls electrons down the chain in an energyyielding tumble • The energy yielded is used to regener ...
... • NADH passes the electrons to the electron transport chain • Unlike an uncontrolled reaction, the electron transport chain passes electrons in a series of steps instead of one explosive reaction • O2 pulls electrons down the chain in an energyyielding tumble • The energy yielded is used to regener ...
Document
... between mitochondria and cytosol • Generation of oxaloacetate from pyruvate or citric acid cycle intermediates occurs only in the mitochondrion. • Enzymes that convert PEP to glucose are cytosolic. • Either oxaloacetate must leave the mitochondrion for conversion to PEP, or the PEP formed must enter ...
... between mitochondria and cytosol • Generation of oxaloacetate from pyruvate or citric acid cycle intermediates occurs only in the mitochondrion. • Enzymes that convert PEP to glucose are cytosolic. • Either oxaloacetate must leave the mitochondrion for conversion to PEP, or the PEP formed must enter ...
2, The Glyoxylate Pathway
... between mitochondria and cytosol • Generation of oxaloacetate from pyruvate or citric acid cycle intermediates occurs only in the mitochondrion. • Enzymes that convert PEP to glucose are cytosolic. • Either oxaloacetate must leave the mitochondrion for conversion to PEP, or the PEP formed must enter ...
... between mitochondria and cytosol • Generation of oxaloacetate from pyruvate or citric acid cycle intermediates occurs only in the mitochondrion. • Enzymes that convert PEP to glucose are cytosolic. • Either oxaloacetate must leave the mitochondrion for conversion to PEP, or the PEP formed must enter ...
HONORS CHEMISTRY
... Ammonia gas produced as a by-product in an industrial reaction can react with sulfuric acid in order that the gas does not escape into the atmosphere. The product, ammonium sulfate, can be used as a fertilizer. Determine how many kilograms of acid are required to produce 1000.0 kilograms of (NH4)2SO ...
... Ammonia gas produced as a by-product in an industrial reaction can react with sulfuric acid in order that the gas does not escape into the atmosphere. The product, ammonium sulfate, can be used as a fertilizer. Determine how many kilograms of acid are required to produce 1000.0 kilograms of (NH4)2SO ...
Final Exam (5/15/14)
... 9. In some bacteria, the citric acid cycle runs backwards from oxaloacetate to citrate to reduce CO2. We feed bacteria with oxaloacetate labeled with14-C on the methyl carbon (-CH2-). a. Draw the citrate molecule indicating where the label is. If only a fraction of molecules contain that label, indi ...
... 9. In some bacteria, the citric acid cycle runs backwards from oxaloacetate to citrate to reduce CO2. We feed bacteria with oxaloacetate labeled with14-C on the methyl carbon (-CH2-). a. Draw the citrate molecule indicating where the label is. If only a fraction of molecules contain that label, indi ...
1 Assignment 4 Hydrogen – The Unique Element
... Both molecular and saline hydrides are quite reactive. Group 1 and 2 hydrides react vigorously with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have dif ...
... Both molecular and saline hydrides are quite reactive. Group 1 and 2 hydrides react vigorously with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have dif ...
1 Assignment 5 Hydrogen – The Unique Element
... Both molecular and saline hydrides are quite reactive. Group 1 and 2 hydrides react vigorously with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have dif ...
... Both molecular and saline hydrides are quite reactive. Group 1 and 2 hydrides react vigorously with water to produce hydrogen gas and a metal hydroxide. This means that they can be used as drying agents for solvents – the most commonly used in this regard is CaH2. p-Block molecular hydrides have dif ...
Slide 1
... AMP (citrate leaks out of the mitochondria when you make lots of it!). This ensures that rates of glycolysis will more or less match rates of oxidative phosphorylation at all rates of ATP demand, at least until rates of glycolysis speed up due to increasing muscle contraction. We must remember that ...
... AMP (citrate leaks out of the mitochondria when you make lots of it!). This ensures that rates of glycolysis will more or less match rates of oxidative phosphorylation at all rates of ATP demand, at least until rates of glycolysis speed up due to increasing muscle contraction. We must remember that ...
9/5/08 Transcript I
... (and now, the quote of the day) “Mother nature is usually very thrifty.” If you’ve got a 3 carbon intermediate in glycolysis and you need a 3 carbon amino acid, it is going to use that because it doesn’t want to waste time and energy using other things. Page 13 of “Overview of Amino Acid Metaboli ...
... (and now, the quote of the day) “Mother nature is usually very thrifty.” If you’ve got a 3 carbon intermediate in glycolysis and you need a 3 carbon amino acid, it is going to use that because it doesn’t want to waste time and energy using other things. Page 13 of “Overview of Amino Acid Metaboli ...
Worksheet - 1 - SunsetRidgeMSBiology
... 2. Primary consumers are animals that eat primary producers; they are also called herbivores (plant-eaters). 3. Secondary consumers eat primary consumers. They are carnivores (meat-eaters) and omnivores (animals that eat both animals and plants). 4. Tertiary consumers eat secondary consumers. 5. Qua ...
... 2. Primary consumers are animals that eat primary producers; they are also called herbivores (plant-eaters). 3. Secondary consumers eat primary consumers. They are carnivores (meat-eaters) and omnivores (animals that eat both animals and plants). 4. Tertiary consumers eat secondary consumers. 5. Qua ...
Muscle Metabolism lecture teacher
... When meat is cooked, some of the proteins in it denature and become opaque, turning red meat pink. At 60 degrees C, the myoglobin itself denatures and becomes tan-coloured, giving well done meat a brownish-grey colour. Freezing for long periods of time can also denature the myoglobin. Finally, curin ...
... When meat is cooked, some of the proteins in it denature and become opaque, turning red meat pink. At 60 degrees C, the myoglobin itself denatures and becomes tan-coloured, giving well done meat a brownish-grey colour. Freezing for long periods of time can also denature the myoglobin. Finally, curin ...
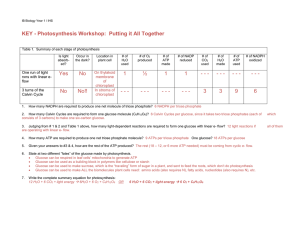
KEY - Photosynthesis Workshop: Putting it All Together
... 2. How many Calvin Cycles are required to form one glucose molecule (C6H12O6)? 6 Calvin Cycles per glucose, since it takes two triose phosphates (each of consists of 3 carbons) to make one six-carbon glucose. ...
... 2. How many Calvin Cycles are required to form one glucose molecule (C6H12O6)? 6 Calvin Cycles per glucose, since it takes two triose phosphates (each of consists of 3 carbons) to make one six-carbon glucose. ...
12_Lecture
... 12.1 How Metabolism Works • Catabolism refers to chemical reactions in which larger molecules are broken down into a few common metabolites. These reactions tend to be exergonic (-G). • Anabolism refers to chemical reactions in which metabolites combine to form larger molecules. These reactions te ...
... 12.1 How Metabolism Works • Catabolism refers to chemical reactions in which larger molecules are broken down into a few common metabolites. These reactions tend to be exergonic (-G). • Anabolism refers to chemical reactions in which metabolites combine to form larger molecules. These reactions te ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)