12.1 Mechanisms regulating enzyme synthesis 12.1.2.2 Enzyme
... Microbial ecosystems are oligotrophic with a limited availability of nutrients. Furthermore, nutrients are not usually found in balanced concentrations while the organisms have to compete with each other for available nutrients. Organic materials are converted to carbon skeletons for monomer a ...
... Microbial ecosystems are oligotrophic with a limited availability of nutrients. Furthermore, nutrients are not usually found in balanced concentrations while the organisms have to compete with each other for available nutrients. Organic materials are converted to carbon skeletons for monomer a ...
electron transport chain
... • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt. • Muscle cells switch from aerobic respiration to lactic acid fermentation to generate ATP when O2 is scarce. • The waste product, lactate, may cause muscle fatigue, but ultimately it is converted back to pyruva ...
... • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt. • Muscle cells switch from aerobic respiration to lactic acid fermentation to generate ATP when O2 is scarce. • The waste product, lactate, may cause muscle fatigue, but ultimately it is converted back to pyruva ...
Module 3- Bioenergetics - Bangen Athletic Development
... The end result of lactate is that it is eventually transported in the blood to the liver, where it is converted to glucose. This process is referred to as the Cori cycle. During aerobic glycolysis, pyruvate is shuttled into the mitochondria of the muscle cell and is converted into a substance known ...
... The end result of lactate is that it is eventually transported in the blood to the liver, where it is converted to glucose. This process is referred to as the Cori cycle. During aerobic glycolysis, pyruvate is shuttled into the mitochondria of the muscle cell and is converted into a substance known ...
Cellular Respiration: Harvesting Chemical Energy
... • The chain’s function is to break the large freeenergy drop from food to O2 into smaller steps that release energy in manageable amounts ...
... • The chain’s function is to break the large freeenergy drop from food to O2 into smaller steps that release energy in manageable amounts ...
lecture9
... Chemical properties of photographic film The film base is usually plastic such as tri-acetate or polyester which is coated with a light sensitive emulsion. The emulsion consists of gelatin containing light sensitive silver halide crystals such as silver bromide and silver chloride. In practice the f ...
... Chemical properties of photographic film The film base is usually plastic such as tri-acetate or polyester which is coated with a light sensitive emulsion. The emulsion consists of gelatin containing light sensitive silver halide crystals such as silver bromide and silver chloride. In practice the f ...
M01
... - secondary to many genetic / acquired disorders (episodic hypoketotic hypoglycemia, starting in infancy) Carnitine supplementation : supposed to increase energy production, because it facilitates the FA transport into mitochondria for oxidation, sparing glycogen from the muscles during exercise; co ...
... - secondary to many genetic / acquired disorders (episodic hypoketotic hypoglycemia, starting in infancy) Carnitine supplementation : supposed to increase energy production, because it facilitates the FA transport into mitochondria for oxidation, sparing glycogen from the muscles during exercise; co ...
Catalytic Nitrene Transfer onto Isocyanide by a Redox
... In an effort to bridge the gap between late- and early-metal reactivity, we have used redox-active ligands that are capable of multielectron valence changes. These ligands on formally d0 metal centers have been shown to facilitate “oxidative addition” of halogens and reductive coupling of C-C and N= ...
... In an effort to bridge the gap between late- and early-metal reactivity, we have used redox-active ligands that are capable of multielectron valence changes. These ligands on formally d0 metal centers have been shown to facilitate “oxidative addition” of halogens and reductive coupling of C-C and N= ...
Introduction to Cell Symbiosis Therapy
... the development of eukaryotes – cells with a nucleus and organelles – was that the archaea engulfed α-proteobacteria, which already at that time had a functioning electron transport chain, so could metabolise oxygen3. These bacteria enabled the archaea to survive in areas where oxygen levels were hi ...
... the development of eukaryotes – cells with a nucleus and organelles – was that the archaea engulfed α-proteobacteria, which already at that time had a functioning electron transport chain, so could metabolise oxygen3. These bacteria enabled the archaea to survive in areas where oxygen levels were hi ...
senatus, miriam
... Because the properties of life is so complicated it is very for scientist to understand biological process. However they emerge by the interactions between components. 4.Describe seven emergent properties associated with life. 1.Order-All characteristics of life emerge from an organism's complex org ...
... Because the properties of life is so complicated it is very for scientist to understand biological process. However they emerge by the interactions between components. 4.Describe seven emergent properties associated with life. 1.Order-All characteristics of life emerge from an organism's complex org ...
2.1 Chemistry’s Building Block: The Atom
... NAD • Through a redox reaction, NAD+ picks up one hydrogen atom and another single electron from food, thus becoming NADH. • It will retain this form until it drops off its energetic electrons (and a proton) in a later stage of the energy-harvesting process. ...
... NAD • Through a redox reaction, NAD+ picks up one hydrogen atom and another single electron from food, thus becoming NADH. • It will retain this form until it drops off its energetic electrons (and a proton) in a later stage of the energy-harvesting process. ...
Unit 10: Protein Catabolism - Central New Mexico Community College
... group from amino acids by breaking the bond between the central carbon and the carboxyl group. (See Figure 10-2 for examples of general enzyme cut sites.) The specific enzyme lysine decarboxylase, removes the carboxyl group from the amino acid lysine. Deaminases are enzymes that remove a molecule’s ...
... group from amino acids by breaking the bond between the central carbon and the carboxyl group. (See Figure 10-2 for examples of general enzyme cut sites.) The specific enzyme lysine decarboxylase, removes the carboxyl group from the amino acid lysine. Deaminases are enzymes that remove a molecule’s ...
Chapter 11
... • Remember: Rubisco normally has carboxylase activity • It also has oxygenase activity • Produces 2 carbon molecule: phosphoglycolate • Phosphoglycolate cannot be used in Calvin Cycle • Why would evolution favor this? • How do plants deal with it? ...
... • Remember: Rubisco normally has carboxylase activity • It also has oxygenase activity • Produces 2 carbon molecule: phosphoglycolate • Phosphoglycolate cannot be used in Calvin Cycle • Why would evolution favor this? • How do plants deal with it? ...
Unit IX - Ecology - Lesson Module
... Parasitism is a symbiotic relationship in which one organism (the parasite) benefits at the expense of the other organism (the host). In general, the parasite does not kill the host. Some parasites, such as tape worms, heartworms, or bacteria, live within the host. Some parasites, such as aphids ...
... Parasitism is a symbiotic relationship in which one organism (the parasite) benefits at the expense of the other organism (the host). In general, the parasite does not kill the host. Some parasites, such as tape worms, heartworms, or bacteria, live within the host. Some parasites, such as aphids ...
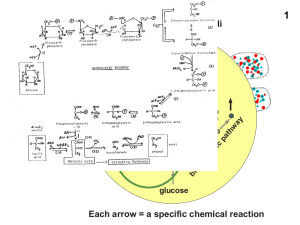
Glucose-6-P to Fructose-6-P
... These two ATP (from one glucose) can be viewed as the "payoff" of glycolysis Large, negative G - regulation! Allosterically activated by AMP, F-1,6-bisP Allosterically inhibited by ATP and acetyl-CoA ...
... These two ATP (from one glucose) can be viewed as the "payoff" of glycolysis Large, negative G - regulation! Allosterically activated by AMP, F-1,6-bisP Allosterically inhibited by ATP and acetyl-CoA ...
Rhodospirillum sulfurexigens sp. nov., a phototrophic
... carotenoids of the spirilloxanthin series with rhodovibrin are present as photosynthetic pigments. Thiamine and a reduced sulfur source are required for growth. Phylogenetic analysis on the basis of 16S rRNA gene sequences showed that strain JA143T clusters with species of the genus Rhodospirillum, ...
... carotenoids of the spirilloxanthin series with rhodovibrin are present as photosynthetic pigments. Thiamine and a reduced sulfur source are required for growth. Phylogenetic analysis on the basis of 16S rRNA gene sequences showed that strain JA143T clusters with species of the genus Rhodospirillum, ...
Document
... But all this spewing turns out to be wasteful. Glucose could be completely oxidized, to: … CO2 That is, burned. How much energy released then? Glucose + 6 O2 6 CO2 + 6 H2O ΔGo = -686 kcal/mole ! Compared to -45 to lactate (both w/o/ ATP considered) Complete oxidation of glucose, Much more ATP But ...
... But all this spewing turns out to be wasteful. Glucose could be completely oxidized, to: … CO2 That is, burned. How much energy released then? Glucose + 6 O2 6 CO2 + 6 H2O ΔGo = -686 kcal/mole ! Compared to -45 to lactate (both w/o/ ATP considered) Complete oxidation of glucose, Much more ATP But ...
Ecology 1 - New Jersey Institute of Technology
... 2. Primary consumers are animals that eat primary producers; they are also called herbivores (plant-eaters). 3. Secondary consumers eat primary consumers. They are carnivores (meat-eaters) and omnivores (animals that eat both animals and plants). 4. Tertiary consumers eat secondary consumers. 5. Qua ...
... 2. Primary consumers are animals that eat primary producers; they are also called herbivores (plant-eaters). 3. Secondary consumers eat primary consumers. They are carnivores (meat-eaters) and omnivores (animals that eat both animals and plants). 4. Tertiary consumers eat secondary consumers. 5. Qua ...
Anaerobic Energy Systems
... If the activity is short duration (less than 10 seconds) and high intensity, we use the ATP–PC system. If the activity is longer than 10 seconds and up to 3 minutes at high intensity, we use the lactic acid system If the activity is long duration and submaximal pace, we use the aerobic system. ...
... If the activity is short duration (less than 10 seconds) and high intensity, we use the ATP–PC system. If the activity is longer than 10 seconds and up to 3 minutes at high intensity, we use the lactic acid system If the activity is long duration and submaximal pace, we use the aerobic system. ...
(ATP). - WordPress.com
... In stage 3 of catabolism, the citric acid cycle is a series of reactions connects the intermediate acetyl CoA from the metabolic pathways in stages 1 and 2 with electron transport and the synthesis of ATP in stage 3 operates under aerobic conditions only oxidizes the two-carbon acetyl group ...
... In stage 3 of catabolism, the citric acid cycle is a series of reactions connects the intermediate acetyl CoA from the metabolic pathways in stages 1 and 2 with electron transport and the synthesis of ATP in stage 3 operates under aerobic conditions only oxidizes the two-carbon acetyl group ...
Environmental adaptation to lagoon systems
... and Khlebovich (1969) later expanded this concept by incorporating much more data. Pronounccd physiotogical changes occur within this critieal salinity boundary region, including distortion of cellular electrochemical properties, tissue albumin fraction alterations, and changcs in growth, locomotion ...
... and Khlebovich (1969) later expanded this concept by incorporating much more data. Pronounccd physiotogical changes occur within this critieal salinity boundary region, including distortion of cellular electrochemical properties, tissue albumin fraction alterations, and changcs in growth, locomotion ...
Input - CBSD.org
... Review Question 2 • How does a noncompetitive inhibitor affect enzyme activity? – Noncompetitive inhibitors bind to a location other than the active site. Their binding changes the shape of the enzyme making normal substrate ...
... Review Question 2 • How does a noncompetitive inhibitor affect enzyme activity? – Noncompetitive inhibitors bind to a location other than the active site. Their binding changes the shape of the enzyme making normal substrate ...
Glucose-6-P to Fructose-6-P
... These two ATP (from one glucose) can be viewed as the "payoff" of glycolysis Large, negative G - regulation! Allosterically activated by AMP, F-1,6-bisP Allosterically inhibited by ATP and acetyl-CoA ...
... These two ATP (from one glucose) can be viewed as the "payoff" of glycolysis Large, negative G - regulation! Allosterically activated by AMP, F-1,6-bisP Allosterically inhibited by ATP and acetyl-CoA ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)