CP CHEMISTRY STUDY GUIDE
... PA Academic Standards - Science & Technology 3.1.10.C. Apply and assess patterns as repeated processes or recurring elements in science and technology. 3.4.10/12.A. Explain and apply concepts about the structure and properties of matter. ...
... PA Academic Standards - Science & Technology 3.1.10.C. Apply and assess patterns as repeated processes or recurring elements in science and technology. 3.4.10/12.A. Explain and apply concepts about the structure and properties of matter. ...
Grouping of Elements in the Periodic Table
... 7. Which elements are most likely to lose electrons and form cations? a) transition metals b) noble gases c) elements in the last two periods d) metals in the first two periods 8. What is another name for semimetals? a) alkaline earth metals b) alkali metals c) transition metals d) metalloids 9. How ...
... 7. Which elements are most likely to lose electrons and form cations? a) transition metals b) noble gases c) elements in the last two periods d) metals in the first two periods 8. What is another name for semimetals? a) alkaline earth metals b) alkali metals c) transition metals d) metalloids 9. How ...
Chemistry
... volume of a material. The formula for calculating density is density = mass divided by volume, D= m/v. The common units of density are grams per cubic centimeter, g/cm or grams per unit milliliter, g/ml. Compound – when two or more elements combine chemically and form a new substance. Compounds have ...
... volume of a material. The formula for calculating density is density = mass divided by volume, D= m/v. The common units of density are grams per cubic centimeter, g/cm or grams per unit milliliter, g/ml. Compound – when two or more elements combine chemically and form a new substance. Compounds have ...
3.08_Periodic Table and the Atom
... 22. Elements of Groups 17 are called ________________________________. 23. The most active element in Group 17 is ________________________________. 24. Elements of Groups 18 are called ________________________________. 25. What sublevels are filling across the Transition Metals? ________________ 26. ...
... 22. Elements of Groups 17 are called ________________________________. 23. The most active element in Group 17 is ________________________________. 24. Elements of Groups 18 are called ________________________________. 25. What sublevels are filling across the Transition Metals? ________________ 26. ...
Atomic Theory and the Periodic Table Video Questions
... What did Meneleev order the elements by? What did Moseley order the elements by? What is the chemical symbol for magnesium? Where is one place the compounds of potassium are found? What is used in a pencil for writing? What do we call atoms of the same element with different numbers of neutrons? Wha ...
... What did Meneleev order the elements by? What did Moseley order the elements by? What is the chemical symbol for magnesium? Where is one place the compounds of potassium are found? What is used in a pencil for writing? What do we call atoms of the same element with different numbers of neutrons? Wha ...
Section 5.1 Review
... 1. _____ In the modern periodic table, elements are ordered (a) according to decreasing atomic mass. (b) according to Mendeleev’s original design. (c) according to increasing atomic number. (d) based on when they were discovered. 2. _____ Mendeleev noticed that certain similarities in the chemical p ...
... 1. _____ In the modern periodic table, elements are ordered (a) according to decreasing atomic mass. (b) according to Mendeleev’s original design. (c) according to increasing atomic number. (d) based on when they were discovered. 2. _____ Mendeleev noticed that certain similarities in the chemical p ...
http://www.sps186.org/downloads/attachments/36092/Periodic%20Table%20Worksheet.pdf
... 3. Elements in the same row across belong to the same _______________________________. 4-5. Elements in the same column down belong to the same ______________or______________. 6. Elements in the same group or family share important ______________________________. 7-9. An element is identified as eit ...
... 3. Elements in the same row across belong to the same _______________________________. 4-5. Elements in the same column down belong to the same ______________or______________. 6. Elements in the same group or family share important ______________________________. 7-9. An element is identified as eit ...
Me, Myself, I, Chlorine BY: Ethan. BP:2
... attaching to other elements I am actually very useful. I could be found in many things but with another element attached to me. I can be found in bleach, paint thinner and pool cleaner just to name a few. You see i’m actually very popular around the united states of america. I’m in the top 10 elemen ...
... attaching to other elements I am actually very useful. I could be found in many things but with another element attached to me. I can be found in bleach, paint thinner and pool cleaner just to name a few. You see i’m actually very popular around the united states of america. I’m in the top 10 elemen ...
Page|1 - askIITians
... Q26. What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group? ...
... Q26. What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group? ...
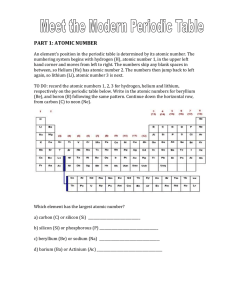
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
SOL Review Station: Equipment, Accuracy, Precision and Lab Safety
... 6. Define the following and describe what happens to the following as you go across and down the Periodic Table? a. Electronegativity ...
... 6. Define the following and describe what happens to the following as you go across and down the Periodic Table? a. Electronegativity ...
The Periodic Table - Mr Linseman`s wiki
... are soluble in water (group 1) Alkaline earth metals: shiny, silvery metals, form compounds that are often insoluble in water (group 2) Halogens: poisonous, react readily with alkali metals (group 17) Noble gases: do not form compounds (have a full outer shell) (group 18) Periods: The rows of ...
... are soluble in water (group 1) Alkaline earth metals: shiny, silvery metals, form compounds that are often insoluble in water (group 2) Halogens: poisonous, react readily with alkali metals (group 17) Noble gases: do not form compounds (have a full outer shell) (group 18) Periods: The rows of ...
6-Getting to Know the Periodic Table
... 1) Using blue ink, write in the group numbers of each group below. Label the following families: Alkali Metals, Alkaline Earth Metals, Halogens and Noble Gases. 2) Using red ink, show the lewis dot structure for groups 1-2, 13-18. 3) Using black ink OR pencil, write in the period number for each per ...
... 1) Using blue ink, write in the group numbers of each group below. Label the following families: Alkali Metals, Alkaline Earth Metals, Halogens and Noble Gases. 2) Using red ink, show the lewis dot structure for groups 1-2, 13-18. 3) Using black ink OR pencil, write in the period number for each per ...
noble gases
... Hydrogen is a unique element (yellow). It’s most common isotope has only a single proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination wit ...
... Hydrogen is a unique element (yellow). It’s most common isotope has only a single proton and no neutron in its nucleus. Hydrogen doesn’t have much in common with the alkali metals. It’s a colourless, odourless, tasteless, highly flammable gas. Almost all of Earth’s hydrogen exists in combination wit ...
Periodic Table Test Chemistry 1 1. What is the horizontal row in the
... Periodic Table Test Chemistry 1 1. What is the horizontal row in the periodic table? 2. What is the vertical column in the periodic table? 3. What states that the repetition of properties occurs when elements are arranged in order of increasing atomic number? 4. What type of element is a good conduc ...
... Periodic Table Test Chemistry 1 1. What is the horizontal row in the periodic table? 2. What is the vertical column in the periodic table? 3. What states that the repetition of properties occurs when elements are arranged in order of increasing atomic number? 4. What type of element is a good conduc ...
Trends in Atomic Radii – Visualization Activity
... The reactivity of an atom depends on how easily the _____________________ electrons can be removed from or attracted to the atom, and that depends on their distance from the attractive force of the __________________. The further away electrons are from the nucleus, the ________________ eas ...
... The reactivity of an atom depends on how easily the _____________________ electrons can be removed from or attracted to the atom, and that depends on their distance from the attractive force of the __________________. The further away electrons are from the nucleus, the ________________ eas ...
Atomic Structure and The Periodic Table
... How does the atomic structure determine the properties of elements and their positions on the periodic table? ...
... How does the atomic structure determine the properties of elements and their positions on the periodic table? ...
The Periodic Table
... (hint: look in the fourth and the sixth periods ) 6. Name three elements which are found in more than one form. _____ _____ _____ 7. Name two metals which are not silver in colour. ___________ ___________ 8. Which element is used in the manufacture of light bulbs? __________ 9. Which is the only liq ...
... (hint: look in the fourth and the sixth periods ) 6. Name three elements which are found in more than one form. _____ _____ _____ 7. Name two metals which are not silver in colour. ___________ ___________ 8. Which element is used in the manufacture of light bulbs? __________ 9. Which is the only liq ...
The Periodic Table assignment
... Name__________________________________ period _____ date assigned_____________ date due ______________ date returned _____________ ...
... Name__________________________________ period _____ date assigned_____________ date due ______________ date returned _____________ ...
Atomic/Periodic Table Review
... 2. Where is most of the mass of an atom found? 3. What is the overall charge of the nucleus and why? 4. Where are the electrons and what keeps them from flying off? 5. What does the atomic number tell you about an element? 6. What is the difference b/w atomic mass and the mass number? 7. How can you ...
... 2. Where is most of the mass of an atom found? 3. What is the overall charge of the nucleus and why? 4. Where are the electrons and what keeps them from flying off? 5. What does the atomic number tell you about an element? 6. What is the difference b/w atomic mass and the mass number? 7. How can you ...
Atoms, elements and compounds
... Sort the different elements in to groups. Write down each group and the property you grouped the elements by. Write a reason for each of you choices. Look at a copy of the periodic table. Use the table and other sources of information to fill in the missing information on your element data cards ...
... Sort the different elements in to groups. Write down each group and the property you grouped the elements by. Write a reason for each of you choices. Look at a copy of the periodic table. Use the table and other sources of information to fill in the missing information on your element data cards ...