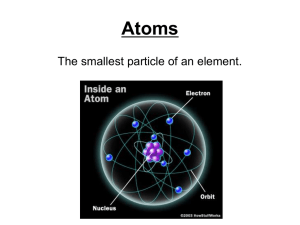
Name Period
... 1. Sodium (Na) and calcium (Ca) are in different families (groups) of metals. Name the families of metals in which they belong and describe each family’s characteristics. Na ---family name_____________number__ Characteristics of that family:______________________________ ____________________________ ...
... 1. Sodium (Na) and calcium (Ca) are in different families (groups) of metals. Name the families of metals in which they belong and describe each family’s characteristics. Na ---family name_____________number__ Characteristics of that family:______________________________ ____________________________ ...
eighth/homework2016-17/homework 19
... _____________________ 8. Formed when an atom loses or gains one or more electrons. ______________ 9. It’s the average mass of the isotopes of atoms of an element. _____________ 10. A table showing a repeating pattern of properties of the elements. _____________________ 11. The elements in a column i ...
... _____________________ 8. Formed when an atom loses or gains one or more electrons. ______________ 9. It’s the average mass of the isotopes of atoms of an element. _____________ 10. A table showing a repeating pattern of properties of the elements. _____________________ 11. The elements in a column i ...
ELEMENTS and THEIR PROPERTIES
... Transition Elements- they often occur in nature as uncombined elements • Typically form colored compounds- chromium found in rubies and emeralds • Iron triad- iron, cobalt, and nickel iron- most widely used of all metals and main ingredient in steel; abundant in Earth’s crust Cobalt and Nickel- use ...
... Transition Elements- they often occur in nature as uncombined elements • Typically form colored compounds- chromium found in rubies and emeralds • Iron triad- iron, cobalt, and nickel iron- most widely used of all metals and main ingredient in steel; abundant in Earth’s crust Cobalt and Nickel- use ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
File
... found on the right hand side of the periodic table__________________________ make up the majority of the periodic table__________________________ **Use the figure below to answer questions 27-30** 27. Which elements have the same number of ...
... found on the right hand side of the periodic table__________________________ make up the majority of the periodic table__________________________ **Use the figure below to answer questions 27-30** 27. Which elements have the same number of ...
Colored Period Table
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
Atoms and Periodic Table
... start with actinium (Ac) at atomic number 89 and finishing up with lawrencium (Lr) at number 103. • They are all radioactive and some are not found in nature. ...
... start with actinium (Ac) at atomic number 89 and finishing up with lawrencium (Lr) at number 103. • They are all radioactive and some are not found in nature. ...
Chapter 5
... 1. Which element above has the highest atomic number? Barium, 56 2. Which element above has the lowest atomic mass? Magnesium, 12 ...
... 1. Which element above has the highest atomic number? Barium, 56 2. Which element above has the lowest atomic mass? Magnesium, 12 ...
Review: Atomic structure/Periodic Table
... Read/interpret information from the periodic table (atomic mass, name, symbol, atomic number) Determine the number of protons, neutrons and electrons for any element Relate the organization of the Periodic Table to the arrangement of electrons within an atom Explain the relationship between ...
... Read/interpret information from the periodic table (atomic mass, name, symbol, atomic number) Determine the number of protons, neutrons and electrons for any element Relate the organization of the Periodic Table to the arrangement of electrons within an atom Explain the relationship between ...
Chapter 8 Study Guide
... Periodic Law- properties of elements change periodically with the elements’ atomic number (Now the periodic table is set up by increasing atomic number) Family/Group –(8) columns of the periodic table; elements are similar in same family Period- (7) rows of the periodic table; elements are NOT simil ...
... Periodic Law- properties of elements change periodically with the elements’ atomic number (Now the periodic table is set up by increasing atomic number) Family/Group –(8) columns of the periodic table; elements are similar in same family Period- (7) rows of the periodic table; elements are NOT simil ...
Chapter 5: Atomic Structure and The Periodic Table
... Be able to read the periodic table and find the atomic number, element name, element symbol, and atomic mass of an element. ...
... Be able to read the periodic table and find the atomic number, element name, element symbol, and atomic mass of an element. ...
Physical properties
... substance reflects light; a dull luster means that the substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physical property of metals that allows them to be drawn out into a wire a physical property that allows substances to break or shatter ...
... substance reflects light; a dull luster means that the substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physical property of metals that allows them to be drawn out into a wire a physical property that allows substances to break or shatter ...
Groups of the Periodic Table
... 16. What is the difference between an electrical conductor and a thermal conductor? ...
... 16. What is the difference between an electrical conductor and a thermal conductor? ...
Science Review Sheet: Periodic Table Test Name: __________
... 12. What are the columns on the periodic table called? What do the elements in each column of the periodic table have in common with each other? ...
... 12. What are the columns on the periodic table called? What do the elements in each column of the periodic table have in common with each other? ...
Chapter 6 Periodic law- states that when the elements are arranged
... Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic table Representative element- groups of elements in the modern periodic table that are designated with an A (1A-8A) and possess a wide range of chemical and ph ...
... Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic table Representative element- groups of elements in the modern periodic table that are designated with an A (1A-8A) and possess a wide range of chemical and ph ...
Reading the Periodic Table
... •ductile and malleable, and conduct electricity and heat •iron, cobalt, and nickel, are the only elements known to produce a magnetic field. ...
... •ductile and malleable, and conduct electricity and heat •iron, cobalt, and nickel, are the only elements known to produce a magnetic field. ...
Notes 3-2
... Ductile – a term used to describe a material that can be pulled out into a long wire. Conductor – a substance that transmits heat or electricity easily. Magnetic – a characteristic of metals in which it is attracted to magnets or can be made into a magnet. Chemical Properties of Metals Reactivity – ...
... Ductile – a term used to describe a material that can be pulled out into a long wire. Conductor – a substance that transmits heat or electricity easily. Magnetic – a characteristic of metals in which it is attracted to magnets or can be made into a magnet. Chemical Properties of Metals Reactivity – ...
Periodic Table Patterns -text 133
... 13.Circle the letter of the one statement that is true about elements in each group. a.They all have the same atomic mass. b.They all have similar characteristics. c.They all have similar atomic numbers. d.They all have the same chemical symbol. 14.The atomic number for the element calcium (Ca) is 2 ...
... 13.Circle the letter of the one statement that is true about elements in each group. a.They all have the same atomic mass. b.They all have similar characteristics. c.They all have similar atomic numbers. d.They all have the same chemical symbol. 14.The atomic number for the element calcium (Ca) is 2 ...
Periodic Table Notes Page
... The elements are listed on the Periodic Table in _____________________ ________________, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s ___________________ is based on the number of protons in each ...
... The elements are listed on the Periodic Table in _____________________ ________________, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s ___________________ is based on the number of protons in each ...
Document
... 17. The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the a. proton. c. electron. b. neutron. d. photon. 18. Which of the following atoms would you expect to have the largest atomic radius? a. I c. Ca b. K d. Rb 19. From left to ...
... 17. The subatomic particle that plays the greatest role in determining the physical and chemical properties of an element is the a. proton. c. electron. b. neutron. d. photon. 18. Which of the following atoms would you expect to have the largest atomic radius? a. I c. Ca b. K d. Rb 19. From left to ...
Unit 3 Practice Test
... 2. While exploring the bottom of the ocean floor, you come across a very cool element that you need to identify. After running some laboratory tests, you determine that the element is a gas at room temperature, doesn’t bind with any other elements, and doesn’t conduct electricity. a. Do you think it ...
... 2. While exploring the bottom of the ocean floor, you come across a very cool element that you need to identify. After running some laboratory tests, you determine that the element is a gas at room temperature, doesn’t bind with any other elements, and doesn’t conduct electricity. a. Do you think it ...
The Periodic Table Chemistry – Leaving Cert Quick Notes
... Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of those whose atoms have the same atomic number. Dobereiner suggested elements of similar properties could be arranged in g ...
... Davy isolated potassium and sodium from their hydroxide compounds. Henry Moseley used the concept of atomic number to derive another definition for an element: a substance all of those whose atoms have the same atomic number. Dobereiner suggested elements of similar properties could be arranged in g ...
Periodic Table cloze activity.
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
Elements and the Periodic Table Section One
... Malleable: a term used to describe material that can be hammered or rolled into shape (pg. ...
... Malleable: a term used to describe material that can be hammered or rolled into shape (pg. ...