ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), molecular oxygen (O2) and molecular nitrogen (N2) are not compou ...
... • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), molecular oxygen (O2) and molecular nitrogen (N2) are not compou ...
PeriodicTableNotes
... Every periodic table will have a square for each element with the atomic number, atomic mass, element name, and the element symbol. o The _______________ _______________ is the symbol given to represent that particular element of that square. o The ______________ ________________ is the actual name ...
... Every periodic table will have a square for each element with the atomic number, atomic mass, element name, and the element symbol. o The _______________ _______________ is the symbol given to represent that particular element of that square. o The ______________ ________________ is the actual name ...
Unit 10: Chemical Periodicity
... Part B True-False--Use AT (always true), ST (sometimes true) or NT (never true) 11. ____________ The representative elements include the halogens. 12. ____________ Chlorine has the electron configuration 1s22s22p63s23p7. 13. ____________ The element in Group 14, period 3, is gallium. 14. ___________ ...
... Part B True-False--Use AT (always true), ST (sometimes true) or NT (never true) 11. ____________ The representative elements include the halogens. 12. ____________ Chlorine has the electron configuration 1s22s22p63s23p7. 13. ____________ The element in Group 14, period 3, is gallium. 14. ___________ ...
Structure of Atoms/Periodic Table Review 1. Shade in location of the
... 2. How is the modern periodic table organized? 3. Who created the first periodic table? 4. What can you predict about an element based on where it is on the periodic table? ...
... 2. How is the modern periodic table organized? 3. Who created the first periodic table? 4. What can you predict about an element based on where it is on the periodic table? ...
Periodic Table Notes The Periodic Table Use Resource #1
... Periodic Table Notes The Periodic Table Use Resource #1 to complete this section. Define and describe: period – ...
... Periodic Table Notes The Periodic Table Use Resource #1 to complete this section. Define and describe: period – ...
Section 14.2 - CPO Science
... • Because they are so different from metals, elements on the far right of the table are called non-metals. • Nonmetals make good insulators. • An insulator is a material which slows down or stops the flow of either heat or electricity. ...
... • Because they are so different from metals, elements on the far right of the table are called non-metals. • Nonmetals make good insulators. • An insulator is a material which slows down or stops the flow of either heat or electricity. ...
Periodic Table Notes Odysseyware Vocabulary the average relative
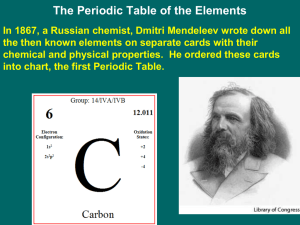
... In the late ______________the sixty-three known elements had been classified according to their properties, but they hadn’t been organized. Then, he (Mendeleev) began to lay the cards out according to increasing ________________ ____________________. The Modern Periodic Table Notice that the element ...
... In the late ______________the sixty-three known elements had been classified according to their properties, but they hadn’t been organized. Then, he (Mendeleev) began to lay the cards out according to increasing ________________ ____________________. The Modern Periodic Table Notice that the element ...
MS Word Printable
... 5. Find potassium. What is the atomic #? ______ atomic mass? ______ # of neutrons? _____ 6. In an electrically neutral carbon atom, how many protons are present? ____ how many electrons? _____ 7. The elements in the far right column, group 8 or VIII, are considered stable in terms of bonding. Name t ...
... 5. Find potassium. What is the atomic #? ______ atomic mass? ______ # of neutrons? _____ 6. In an electrically neutral carbon atom, how many protons are present? ____ how many electrons? _____ 7. The elements in the far right column, group 8 or VIII, are considered stable in terms of bonding. Name t ...
Elements and the Periodic Table
... 21. Looking at the periodic table, which element would have similar chemical properties to Beryllium, Be? 22. Complete the following chart: Element Symbol Atomic ...
... 21. Looking at the periodic table, which element would have similar chemical properties to Beryllium, Be? 22. Complete the following chart: Element Symbol Atomic ...
Activity - Periodic Table Scavenger Hunt
... Without repeating the elements you found above, answer the following questions: 3. Find three elements in the same family ...
... Without repeating the elements you found above, answer the following questions: 3. Find three elements in the same family ...
Science Review Sheet: Periodic Table Test Name: ______ Study
... Study periodic table notes. Know the properties of Alkali Metals, Alkaline Earth Metals, Halides/Halogens and Noble Gases. Know how to calculate atomic mass, # of protons, # of electrons, and #of neutrons in an atom. 1. What are the three subatomic particles? Where are they found within an atom? Wha ...
... Study periodic table notes. Know the properties of Alkali Metals, Alkaline Earth Metals, Halides/Halogens and Noble Gases. Know how to calculate atomic mass, # of protons, # of electrons, and #of neutrons in an atom. 1. What are the three subatomic particles? Where are they found within an atom? Wha ...
Chapter 12 The Periodic Table
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
2.2 Periodic Chart
... (1- in charge), then it becomes a +1 ion. A charged atom or group of atoms is called an ion. The ion charge is the electric charge that forms when an atom gains or loses electrons to become more stable. ...
... (1- in charge), then it becomes a +1 ion. A charged atom or group of atoms is called an ion. The ion charge is the electric charge that forms when an atom gains or loses electrons to become more stable. ...
Date Period - Swift Classroom
... He put the elements in order by _______________________ He found that other properties such as _________________________, _______________________, and the ability to ______________________ with other elements seemed to ____________________ over and over. This repeating pattern is called ______ ...
... He put the elements in order by _______________________ He found that other properties such as _________________________, _______________________, and the ability to ______________________ with other elements seemed to ____________________ over and over. This repeating pattern is called ______ ...
In the space provided, write the letter of the term or phrase that best
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
... ______ 5. Nonmetallic elements in Group 17 that react with most metals to form salts are a. alkali metals. b. halogens. c. lanthanides. d. noble gases. ______ 6. The outer-level electron configuration of a neutral alkaline-earth metal atom consists of a. one electron in the s orbital. b. two electro ...
2012 chapter 4 study guide
... The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a basis for understanding this concept: 7 a. Students know how to identify regions corresponding to metals, nonmetals, and inert gases. Be able to 7. tell who Mendeleev was and h ...
... The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a basis for understanding this concept: 7 a. Students know how to identify regions corresponding to metals, nonmetals, and inert gases. Be able to 7. tell who Mendeleev was and h ...
Study Guide - Chapter 12 Quiz
... C. most are solid at room temperature Nonmetals A. found to the right of the zigzag line B. most have an almost complete set of electrons in the outer level C. More than half are gases at room temperature Metalloids - also called semiconductors A. border the zigzag line B. about half of a complete s ...
... C. most are solid at room temperature Nonmetals A. found to the right of the zigzag line B. most have an almost complete set of electrons in the outer level C. More than half are gases at room temperature Metalloids - also called semiconductors A. border the zigzag line B. about half of a complete s ...
The Periodic Table
... Henry Moseley changed Mendeleev’s periodic table and put the atoms in order according to increasing atomic number (protons) This fixed the problem. Now all of the elements fell into place ...
... Henry Moseley changed Mendeleev’s periodic table and put the atoms in order according to increasing atomic number (protons) This fixed the problem. Now all of the elements fell into place ...
Chemistry Study Guide - Atomic structure and the Periodic Table 2010
... 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: a. What is the structure of the atom and how are protons, neutrons, and electrons arran ...
... 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: a. What is the structure of the atom and how are protons, neutrons, and electrons arran ...
The Periodic Table
... • The horizontal rows of elements are called Periods • The period tells the number of shells in the atom of an element • The 1st period has 2 elements H and He. • The 2nd period goes from Li to Ne. • The 3rd period goes from Na to Ar. ...
... • The horizontal rows of elements are called Periods • The period tells the number of shells in the atom of an element • The 1st period has 2 elements H and He. • The 2nd period goes from Li to Ne. • The 3rd period goes from Na to Ar. ...