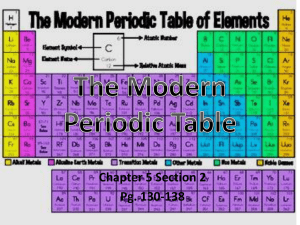
Periodic Table
... Concept: Periodic Table Periodic Table: 1. Contains all known elements 2. Each element has a name, chemical symbol, and atomic number 3. Organized by the elements' chemical property, which is their Atomic Number TPS: In your own words explain how the periodic table is organized. ...
... Concept: Periodic Table Periodic Table: 1. Contains all known elements 2. Each element has a name, chemical symbol, and atomic number 3. Organized by the elements' chemical property, which is their Atomic Number TPS: In your own words explain how the periodic table is organized. ...
Introduction to the Periodic Table
... Introduction to the Periodic Table Atomic Number ● Symbol ● Atomic Weight ...
... Introduction to the Periodic Table Atomic Number ● Symbol ● Atomic Weight ...
Document
... 1. Why are group numbers so important? __________________________________________ _______________________________________________________________________ 2. Why is hydrogen in Group 1 if it is a nonmetal?_____________________________________ __________________________________________________________ ...
... 1. Why are group numbers so important? __________________________________________ _______________________________________________________________________ 2. Why is hydrogen in Group 1 if it is a nonmetal?_____________________________________ __________________________________________________________ ...
Unit 1 Matter: Properties and Change
... Metals are on the left side of the Periodic Table These metals have properties that you normally associate with the ...
... Metals are on the left side of the Periodic Table These metals have properties that you normally associate with the ...
20161025140773
... – To have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard – An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom ...
... – To have a convenient way to compare the masses of atoms, scientists chose one isotope to serve as a standard – An atomic mass unit (amu) is defined as one twelfth the mass of a carbon-12 atom ...
The Periodic Table
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
Test 1. 2nd prep. ques
... Write balanced chemical equation which expresses each of the following reaction 1- Bromine with potassium iodine ----Br2+2 KI ⤍2KBr + I2------------------------------------------------------------------2- Sodium with water ------2Na + 2H2O⇢ 2 NaOH + H2------------------------------------------------ ...
... Write balanced chemical equation which expresses each of the following reaction 1- Bromine with potassium iodine ----Br2+2 KI ⤍2KBr + I2------------------------------------------------------------------2- Sodium with water ------2Na + 2H2O⇢ 2 NaOH + H2------------------------------------------------ ...
The Periodic Table
... 13. A period (a row) on the periodic table has elements that have what in common… A. They have similar chemical properties B. They have the same number of electrons C. They have the same number of electron shells D. They are similarly reactive in compounds. 14. The vertical columns in a periodic tab ...
... 13. A period (a row) on the periodic table has elements that have what in common… A. They have similar chemical properties B. They have the same number of electrons C. They have the same number of electron shells D. They are similarly reactive in compounds. 14. The vertical columns in a periodic tab ...
u4ohnotes18f2005 - Teach-n-Learn-Chem
... in-between those of metals and nonmetals “semiconductors” ...
... in-between those of metals and nonmetals “semiconductors” ...
Chemistry: Unit 4 - Teach-n-Learn-Chem
... in-between those of metals and nonmetals “semiconductors” ...
... in-between those of metals and nonmetals “semiconductors” ...
Reinforcing Key Concepts
... radioactivity by the time it takes for one-half of a sample of atoms to change identity. For example, lead-214 has a half-life of 27 minutes. If you started with 500 grams of this isotope, how many grams would you have after 54 minutes? ...
... radioactivity by the time it takes for one-half of a sample of atoms to change identity. For example, lead-214 has a half-life of 27 minutes. If you started with 500 grams of this isotope, how many grams would you have after 54 minutes? ...
Periodic Table and Elements Review
... 18.) In one of labs we looked investigated the reactivity of aluminum, magnesium, and calcium by immersing them in water. What was the order from most to least reactive? ...
... 18.) In one of labs we looked investigated the reactivity of aluminum, magnesium, and calcium by immersing them in water. What was the order from most to least reactive? ...
Chapter 5
... • The elements within a group have similar _________ • Properties of elements__________ in a predicable way when atomic number are used to arrange elements into groups • The pattern of repeating properties is the _________ • There are ________ groups on the periodic table ...
... • The elements within a group have similar _________ • Properties of elements__________ in a predicable way when atomic number are used to arrange elements into groups • The pattern of repeating properties is the _________ • There are ________ groups on the periodic table ...
Periodic TABLE: Tables: PT, Table S
... group have the same number of valence electrons (helium is an exception) and therefore similar chemical properties. 3.1aaThe succession of elements within the same group demonstrates characteristic trends: differences in atomic radius, ionic radius, electronegativity, first ionization energy, metall ...
... group have the same number of valence electrons (helium is an exception) and therefore similar chemical properties. 3.1aaThe succession of elements within the same group demonstrates characteristic trends: differences in atomic radius, ionic radius, electronegativity, first ionization energy, metall ...
Introduction To The Periodic Table Of The Elements
... React rapidly when exposed to air and water ...
... React rapidly when exposed to air and water ...
Honors Chemistry- Chapter 5 Homework Packet The Periodic Law
... (c) In which corner of the periodic table do the elements have the highest electronegativity? In which corner do they have the lowest electronegativity? ...
... (c) In which corner of the periodic table do the elements have the highest electronegativity? In which corner do they have the lowest electronegativity? ...
alkaline earth metals
... left of the PT and very reactive • Transition metals—near the center of the PT and include copper, gold, silver, and iron • Rare earth metals– in the top row of the 2 rows of metals show outside the main body of the PT • Two bottom rows—separated from the table to save space. ...
... left of the PT and very reactive • Transition metals—near the center of the PT and include copper, gold, silver, and iron • Rare earth metals– in the top row of the 2 rows of metals show outside the main body of the PT • Two bottom rows—separated from the table to save space. ...
The periodic table
... abbreviation. The first letter has to be capitalized. If there is a second letter, it is always lowercase. ...
... abbreviation. The first letter has to be capitalized. If there is a second letter, it is always lowercase. ...
The History of the Modern Periodic Table
... • was so confident in his table that he used it to predict the physical properties of three elements that were ...
... • was so confident in his table that he used it to predict the physical properties of three elements that were ...
L2: The Atom, Standard Notation and Borh
... Use the periodic table (p.647 in your textbook) to help you. The atomic number is on the top left side of each element symbol on the periodic table and the atomic mass is below each element symbol. For example carbon has atomic number 6 and mass of 12. (Note: The mass is rounded to the nearest whole ...
... Use the periodic table (p.647 in your textbook) to help you. The atomic number is on the top left side of each element symbol on the periodic table and the atomic mass is below each element symbol. For example carbon has atomic number 6 and mass of 12. (Note: The mass is rounded to the nearest whole ...
Periodic Table
... bottom) are called a group Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
... bottom) are called a group Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
Alchemy Unit Concepts Review
... f. Metals vs. nonmetals (where does H belong?) Metals are the left side of the periodic table. Nonmetals and H are the right side periodic table. 11. Using your periodic table, what are the trends for … a. atomic number (both across a period and down a group) Atomic number increases as you move left ...
... f. Metals vs. nonmetals (where does H belong?) Metals are the left side of the periodic table. Nonmetals and H are the right side periodic table. 11. Using your periodic table, what are the trends for … a. atomic number (both across a period and down a group) Atomic number increases as you move left ...