Nuclear - Orangefield ISD
... ◦ Electrons are held within atom by attraction to positively charged nucleus ◦ Number of protons equals number of electrons ...
... ◦ Electrons are held within atom by attraction to positively charged nucleus ◦ Number of protons equals number of electrons ...
radioactive decay
... Skin can stop alpha radiation Beta radiation – usually only penetrates 1-2cm beneath the skin Alpha, beta and gamma are “ionizing radiation” – they have enough energy to break bonds in molecules which ionizes them, which makes them unstable, and very reactive inside the organism Find out more at Ion ...
... Skin can stop alpha radiation Beta radiation – usually only penetrates 1-2cm beneath the skin Alpha, beta and gamma are “ionizing radiation” – they have enough energy to break bonds in molecules which ionizes them, which makes them unstable, and very reactive inside the organism Find out more at Ion ...
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER
... when they combine with water. They include lithium, sodium, potassium, rubidium, cesium, and francium. Any of a group of metallic elements that includes beryllium, magnesium, calcium, strontium, barium, and radium. A positively charged particle, indistinguishable from a helium atom nucleus and consi ...
... when they combine with water. They include lithium, sodium, potassium, rubidium, cesium, and francium. Any of a group of metallic elements that includes beryllium, magnesium, calcium, strontium, barium, and radium. A positively charged particle, indistinguishable from a helium atom nucleus and consi ...
Chapter 25 Nuclear Chemistry
... All nuclei contain protons and neutrons (exception - hydrogen-1 has no neutrons). Since protons are positively charged, it would be expected that they would repel and separate, but this does not occur. A force holds them together. The nuclear force is an attractive force that acts between all nuclea ...
... All nuclei contain protons and neutrons (exception - hydrogen-1 has no neutrons). Since protons are positively charged, it would be expected that they would repel and separate, but this does not occur. A force holds them together. The nuclear force is an attractive force that acts between all nuclea ...
Test #5 Review
... Which is larger, fluorine or bromine? bromine (For the same reason – more energy levels.) Why do elements in the same family behave the same? They all have the same number of valence electrons. ...
... Which is larger, fluorine or bromine? bromine (For the same reason – more energy levels.) Why do elements in the same family behave the same? They all have the same number of valence electrons. ...
AC Geophysical Science Study Guide
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
solutions
... Problem 3. Nobel laureate Richard Feynman once said that if two persons stood at arm’s length from each other and each person had p = 1% more electrons than protons, the force of repulsion between them would be enough to lift a “weight” equal to that of the entire Earth. Carry out an order of magnit ...
... Problem 3. Nobel laureate Richard Feynman once said that if two persons stood at arm’s length from each other and each person had p = 1% more electrons than protons, the force of repulsion between them would be enough to lift a “weight” equal to that of the entire Earth. Carry out an order of magnit ...
worth 50 points!!- Due when you take your midterm!!!
... The atomic mass is the number of protons AND neutrons. A stable atom has equal numbers of protons AND electrons. The modern Periodic Table is arranged how? By increasing atomic number (number of protons) What are isotopes? Atoms with a different number of neutrons than listed on the periodic table. ...
... The atomic mass is the number of protons AND neutrons. A stable atom has equal numbers of protons AND electrons. The modern Periodic Table is arranged how? By increasing atomic number (number of protons) What are isotopes? Atoms with a different number of neutrons than listed on the periodic table. ...
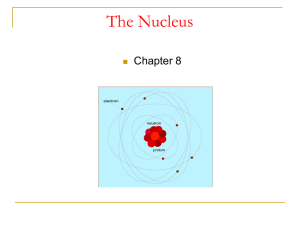
CH_8_nucleus_new
... The resulting nucleus therefore has LESS mass than the total mass of the particles before interacting. The Binding Energy is the energy equivalent of the missing mass of a nucleus. Greater binding energy means more energy must be supplied to break up the nucleus. Without binding energy, no nuclei mo ...
... The resulting nucleus therefore has LESS mass than the total mass of the particles before interacting. The Binding Energy is the energy equivalent of the missing mass of a nucleus. Greater binding energy means more energy must be supplied to break up the nucleus. Without binding energy, no nuclei mo ...
Vocabulary Cards
... Non-charged particle in the nucleus of an atom. To find the number of neutrons: subtract the atomic number from the atomic mass number. ...
... Non-charged particle in the nucleus of an atom. To find the number of neutrons: subtract the atomic number from the atomic mass number. ...
2nd Semester Review
... 4. Circle the correct atomic particle for each of the following: Defines an atom Protons Neutrons Electrons Isotopes: same type of atom with different number of Protons Neutrons Determines how atoms combine Protons Neutrons Electrons Ions: same type of atom with different number of Protons Neutrons ...
... 4. Circle the correct atomic particle for each of the following: Defines an atom Protons Neutrons Electrons Isotopes: same type of atom with different number of Protons Neutrons Determines how atoms combine Protons Neutrons Electrons Ions: same type of atom with different number of Protons Neutrons ...
Nuclear Chemistry
... The nucleus can ‘capture’ one of its own innerorbital electrons if the atom is unstable due to too many protons. The electron will combine with a proton in the nucleus and form a neutron. The atomic number decreases by one but the mass number stays the same. ...
... The nucleus can ‘capture’ one of its own innerorbital electrons if the atom is unstable due to too many protons. The electron will combine with a proton in the nucleus and form a neutron. The atomic number decreases by one but the mass number stays the same. ...
Document
... ! identification of elements in the “atmosphere” of stars, discovery of helium. • The energies of K" x-rays can be calculated by replacing Z by Z–1 ! identification of some chemical elements for the first time. • Stimulated emission, the laser. Monday, April 5, 2010 ...
... ! identification of elements in the “atmosphere” of stars, discovery of helium. • The energies of K" x-rays can be calculated by replacing Z by Z–1 ! identification of some chemical elements for the first time. • Stimulated emission, the laser. Monday, April 5, 2010 ...