ib atomic and nuclear physics definitions and concepts
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
SIMPLE NUCLEAR REACTIONS
... A neutron is fired at a large nucleus (usually uranium-235). It is absorbed briefly which makes the unstable isotope of uranium-236. This then splits into two or more smaller nuclei releasing neutrons and energy in the process. The products are radioactive. Ex. uranium-235 + neutron [uranium-236] ...
... A neutron is fired at a large nucleus (usually uranium-235). It is absorbed briefly which makes the unstable isotope of uranium-236. This then splits into two or more smaller nuclei releasing neutrons and energy in the process. The products are radioactive. Ex. uranium-235 + neutron [uranium-236] ...
IB-ATOMIC-AND-NUCLEAR-PHYSICS-DEFINITIONS
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
... RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disintegrate). The rate decreases exponentially with time. NATURAL RADIOACTIVE DECAY: The emission of an alpha or beta particle. NUCLEAR STRONG FORCE: The force that holds the particles of a nucleus togethe ...
SMP Quiz Session 1
... 4. Some black holes stars exploded. 5. These elements were made by radioacIvity in the Earth's core. ...
... 4. Some black holes stars exploded. 5. These elements were made by radioacIvity in the Earth's core. ...
Nuclear Chemistry
... • Unstable nuclei are naturally “built wrong” and “fall apart” • An unstable nucleus undergoes transmutation, changing from one element into another – the nucleus changes # of protons! ...
... • Unstable nuclei are naturally “built wrong” and “fall apart” • An unstable nucleus undergoes transmutation, changing from one element into another – the nucleus changes # of protons! ...
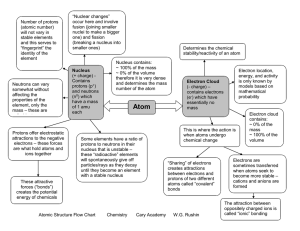
Atomic Structure Flow Chart Chemistry Cary Academy W.G. Rushin
... fusion (joining smaller nuclei to make a bigger one) and fission (breaking a nucleus into smaller ones) ...
... fusion (joining smaller nuclei to make a bigger one) and fission (breaking a nucleus into smaller ones) ...
Atomic Structure and Radioactivity
... - decay: the nucleus composition does not change. -decay occurs in large, unstable, nuclei. The heaviest stable isotope is 20983Bi. -decay transforms a neutron into a proton (n p+ + e ) Electron capture: p+ + e n and p+ n + e+ ...
... - decay: the nucleus composition does not change. -decay occurs in large, unstable, nuclei. The heaviest stable isotope is 20983Bi. -decay transforms a neutron into a proton (n p+ + e ) Electron capture: p+ + e n and p+ n + e+ ...
Document
... mass of one atom of C-12 is exactly 12 u. Mass can also be expressed in MeV/c2 From ER = mc2, 1 u = 931.494 MeV/c2 ...
... mass of one atom of C-12 is exactly 12 u. Mass can also be expressed in MeV/c2 From ER = mc2, 1 u = 931.494 MeV/c2 ...
Name
... 9. The conversion of an atomic nucleus of one element into an atomic nucleus of another element through a loss or gain in the number of protons. 10. High-energy radiation emitted by the nuclei of radioactive atoms. 11. Nuclear fusion produced by high temperature. Down 2. The force of interaction bet ...
... 9. The conversion of an atomic nucleus of one element into an atomic nucleus of another element through a loss or gain in the number of protons. 10. High-energy radiation emitted by the nuclei of radioactive atoms. 11. Nuclear fusion produced by high temperature. Down 2. The force of interaction bet ...