Isotope Half-Life Radiation Emitted
... 1. The nucleus is unstable due to imbalance of protons and neutrons or a large # of neutrons and protons. 2. The strong force (holds the neutrons and protons together) & weak force (holds protons together) are not strong enough. • 3. elements with atomic # > 83 = radioactive ...
... 1. The nucleus is unstable due to imbalance of protons and neutrons or a large # of neutrons and protons. 2. The strong force (holds the neutrons and protons together) & weak force (holds protons together) are not strong enough. • 3. elements with atomic # > 83 = radioactive ...
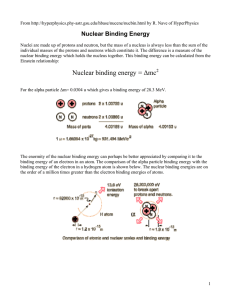
Nuclear binding energy = Δmc2 - University of Toronto Physics
... binding energy of the electron in a hydrogen atom is shown below. The nuclear binding energies are on the order of a million times greater than the electron binding energies of atoms. ...
... binding energy of the electron in a hydrogen atom is shown below. The nuclear binding energies are on the order of a million times greater than the electron binding energies of atoms. ...
Nuclear Chemistry - Northwest ISD Moodle
... Mass number does not change Add 1 to atomic number Identify new element from atomic number Add beta particle ...
... Mass number does not change Add 1 to atomic number Identify new element from atomic number Add beta particle ...
The Atom.jet.2013 - Petal School District
... Represents the number of protons in an atom Example: Carbon (C) has 6 protons Elements differ by their atomic number ...
... Represents the number of protons in an atom Example: Carbon (C) has 6 protons Elements differ by their atomic number ...
Energy per nucleon
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . ...
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . ...
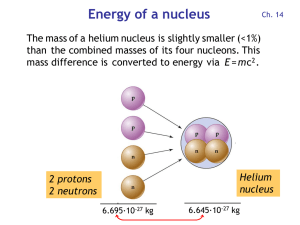
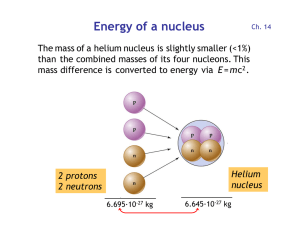
Energy of a nucleus
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nucle ...
... a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nucle ...
1 AP Chemistry Chapter 21 - The Nucleus: A Chemist`s View 21.1
... 1. The minimum amount of nuclide that provides the number of neutrons needed to sustain a chain reaction D. Nuclear Fusion 1. Combining two light nuclei to form a heavier, more stable nucleus ...
... 1. The minimum amount of nuclide that provides the number of neutrons needed to sustain a chain reaction D. Nuclear Fusion 1. Combining two light nuclei to form a heavier, more stable nucleus ...
Accelerated Chemistry: Ch
... Nuclear chemistry is the study of the atomic nucleus and nuclear reactions. A nuclide is an atom consisting of 3 subatomic particles: electrons, protons and neutrons. A nucleon is the nucleus of an atom consisting of neutrons and protons. Keeping the Nucleus Together Two of the four known forces in ...
... Nuclear chemistry is the study of the atomic nucleus and nuclear reactions. A nuclide is an atom consisting of 3 subatomic particles: electrons, protons and neutrons. A nucleon is the nucleus of an atom consisting of neutrons and protons. Keeping the Nucleus Together Two of the four known forces in ...
Chapter 1 Learning Objective Summary
... The atomic weight of an element is what is found on the periodic table, which is actually an average of the atomic masses of all of the existin isotopes of the element, wei hted by their abundances in the earth’s crust. This is called a weighted mean, and can be calculated using fractional abundance ...
... The atomic weight of an element is what is found on the periodic table, which is actually an average of the atomic masses of all of the existin isotopes of the element, wei hted by their abundances in the earth’s crust. This is called a weighted mean, and can be calculated using fractional abundance ...
The New Alchemy
... Protons – one of the parts of an atom. Protons have a (+) charge and are found in the nucleus. Neutrons – one of the parts of an atom. Neutrons have no charge and are found in the nucleus. Nucleus – found in the center of an atom. It contains protons and neutrons. Nuclei is the plural of nucleus. Nu ...
... Protons – one of the parts of an atom. Protons have a (+) charge and are found in the nucleus. Neutrons – one of the parts of an atom. Neutrons have no charge and are found in the nucleus. Nucleus – found in the center of an atom. It contains protons and neutrons. Nuclei is the plural of nucleus. Nu ...
Lecture 30/3 Nuclear processes Ulf Torkelsson 1 Nuclear reactions
... Fe, in which they are the most strongly bound, and in heavier nuclei the binding energy per nucleon decreases with the size of the nucleus. Therefore there are two ways of deriving energy from atomic nuclei, by fusing light nuclei, and by fissioning heavy nuclei. The nuclei that are involved in a fu ...
... Fe, in which they are the most strongly bound, and in heavier nuclei the binding energy per nucleon decreases with the size of the nucleus. Therefore there are two ways of deriving energy from atomic nuclei, by fusing light nuclei, and by fissioning heavy nuclei. The nuclei that are involved in a fu ...
Chapter 21 Powerpoint: Nuclear Chemistry
... 3. Identify the type of particle that has decayed and write it after the arrow. 4. Balance the mass number (top) and the atomic number (bottom). 5. Identify the new element. ...
... 3. Identify the type of particle that has decayed and write it after the arrow. 4. Balance the mass number (top) and the atomic number (bottom). 5. Identify the new element. ...
Radioactivity - Mrs. Sjuts` Science Site
... ! Two positives normally repel each other, so why don’t the protons in the nucleus repel? ! Strong force = one of four basic forces that causes protons and neutrons to be attracted to each other ...
... ! Two positives normally repel each other, so why don’t the protons in the nucleus repel? ! Strong force = one of four basic forces that causes protons and neutrons to be attracted to each other ...