nuclear physics ppt
... Nuclear Reaction: x + X Y + y + Q Conservation of Charge: The total charge of a system can neither be increased nor decreased. Conservation of Nucleons: The total number of nucleons in a reaction must be unchanged. Conservation of Mass Energy: The total massenergy of a system must not change in a ...
... Nuclear Reaction: x + X Y + y + Q Conservation of Charge: The total charge of a system can neither be increased nor decreased. Conservation of Nucleons: The total number of nucleons in a reaction must be unchanged. Conservation of Mass Energy: The total massenergy of a system must not change in a ...
Review 1st Qtr KEY
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
Nuclear Chemistry
... Consists of 2 protons, 2 neutrons emitted during decay Helium nucleus ( 24He )—how particle represented Can be stopped by paper, low energy Atomic number goes down 2, atomic mass goes down 4. ...
... Consists of 2 protons, 2 neutrons emitted during decay Helium nucleus ( 24He )—how particle represented Can be stopped by paper, low energy Atomic number goes down 2, atomic mass goes down 4. ...
2.10 Basic Nuclear Chemistry
... 2. Even numbers tend to be more stable than odd numbers of nucleons. B. As the number of protons in a nucleus increases, the stability of the nucleus decreases 1. This is because the positive repulsive forces are greater than the Nuclear Force. 2. To reduce this instability, neutrons are needed to i ...
... 2. Even numbers tend to be more stable than odd numbers of nucleons. B. As the number of protons in a nucleus increases, the stability of the nucleus decreases 1. This is because the positive repulsive forces are greater than the Nuclear Force. 2. To reduce this instability, neutrons are needed to i ...
Chapter 29
... • Experiments showed that few electrons had this amount of kinetic energy • To account for this “missing” energy, Pauli proposed the existence of another particle, which Fermi later named the neutrino • Properties of the neutrino: ...
... • Experiments showed that few electrons had this amount of kinetic energy • To account for this “missing” energy, Pauli proposed the existence of another particle, which Fermi later named the neutrino • Properties of the neutrino: ...
Exam on Matter through Bonding
... 1. Which electron transition represents a gain of energy? (1) from 2nd to 3rd shell (2) from 2nd to 1st shell (3) from 3rd to 2nd shell (4) from 3rd to 1st shell 2. Which particles are found in the nucleus of an atom? (1) electrons, only (2) neutrons, only (3) protons and electrons (4) protons and n ...
... 1. Which electron transition represents a gain of energy? (1) from 2nd to 3rd shell (2) from 2nd to 1st shell (3) from 3rd to 2nd shell (4) from 3rd to 1st shell 2. Which particles are found in the nucleus of an atom? (1) electrons, only (2) neutrons, only (3) protons and electrons (4) protons and n ...
1 slide per page() - Wayne State University Physics and Astronomy
... Followed by H – He or He – He fusion: or ...
... Followed by H – He or He – He fusion: or ...
I Examen II trim Science
... 13.Explain the relation between the repulsive charges and the strong nuclear force. The strong nuclear force: Is the force that holds together the nucleus. This force acts only in short distances. When there are two much protons, the protons want to escape the nucleus. The neutrons hold together the ...
... 13.Explain the relation between the repulsive charges and the strong nuclear force. The strong nuclear force: Is the force that holds together the nucleus. This force acts only in short distances. When there are two much protons, the protons want to escape the nucleus. The neutrons hold together the ...
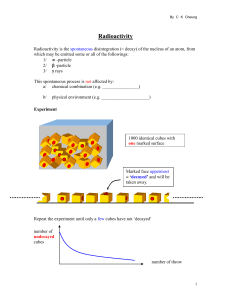
Radioactivity
... minute per gram of carbon. But when the organism dies no intake of C*, but decay process goes on with T1/2 ~ 5700 years. By measuring the activity of some furniture from an ancient village age of the furniture can be estimated within the range of 1000 to 5000 years. ...
... minute per gram of carbon. But when the organism dies no intake of C*, but decay process goes on with T1/2 ~ 5700 years. By measuring the activity of some furniture from an ancient village age of the furniture can be estimated within the range of 1000 to 5000 years. ...
4 slides per page() - Wayne State University Physics and
... In the first atomic bomb, the energy released was equivalent to about 30 kilotons of TNT, where a ton of TNT releases an energy of 4.0 × 109 J. The amount of mass converted into energy in this event is nearest to: (a) 1 μg, (b) 1 mg, (c) 1 g, ...
... In the first atomic bomb, the energy released was equivalent to about 30 kilotons of TNT, where a ton of TNT releases an energy of 4.0 × 109 J. The amount of mass converted into energy in this event is nearest to: (a) 1 μg, (b) 1 mg, (c) 1 g, ...