Chapter 29
... • The emission of the electron is from the nucleus, which contains protons and neutrons • The process occurs when a neutron is transformed into a proton and an electron • The energy must be conserved: the energy released in the decay process should almost all go to kinetic energy of the electron (KE ...
... • The emission of the electron is from the nucleus, which contains protons and neutrons • The process occurs when a neutron is transformed into a proton and an electron • The energy must be conserved: the energy released in the decay process should almost all go to kinetic energy of the electron (KE ...
Nuclear Physics and Radioactivity
... beta particle - high speed electron emitted from a radioactive element when a neutron. decays into a proton binding energy – the energy required to completely separate the nucleus into its individual protons and neutrons. element - a substance made of only one kind of atom. isotope - a form of an el ...
... beta particle - high speed electron emitted from a radioactive element when a neutron. decays into a proton binding energy – the energy required to completely separate the nucleus into its individual protons and neutrons. element - a substance made of only one kind of atom. isotope - a form of an el ...
Modern Physics Important Concepts for AP Test
... Total Energy E = K + mc2 Pair Production and annihilation Process in which a photon creates matter. An electron and positron (positively charged particle with mass of an electron) are produced and the photon disappears. Energy, momentum, and charge must be conserved in this process. Ephoton ...
... Total Energy E = K + mc2 Pair Production and annihilation Process in which a photon creates matter. An electron and positron (positively charged particle with mass of an electron) are produced and the photon disappears. Energy, momentum, and charge must be conserved in this process. Ephoton ...
Review 2 key - Home [www.petoskeyschools.org]
... 12 What are valence electrons? Electrons that are found in the outermost energy level 13 Summarize what happens in a physical change, AND give an example of a physical change In a physical change the substance’s identity is not changed. Examples: cutting, grinding, stretching, ...
... 12 What are valence electrons? Electrons that are found in the outermost energy level 13 Summarize what happens in a physical change, AND give an example of a physical change In a physical change the substance’s identity is not changed. Examples: cutting, grinding, stretching, ...
Unit 14 Notes - shscience.net
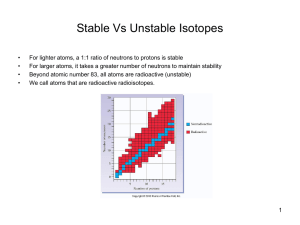
... transferred into stable isotopes of another element. TRANSMUTATION Radioactive decay is spontaneous does not require any input of energy. ...
... transferred into stable isotopes of another element. TRANSMUTATION Radioactive decay is spontaneous does not require any input of energy. ...
Atomic Structure 1. Historical perspective of the model of the atom a
... which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or rearranging atoms. b.) In 1910, Ernest Rutherford pa ...
... which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or rearranging atoms. b.) In 1910, Ernest Rutherford pa ...
File
... Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass d ...
... Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass d ...
PreAP Chemistry Radioactivity WS Name Period ____ Match the
... Match the statement in column A with the correct term from column B. Terms may be used more than once. Column A Column B ---------- 1. This radiation would be deflected towards a positively charged metal plate. A. atom ---------- 2. Atoms with the same number of protons but different numbers of neut ...
... Match the statement in column A with the correct term from column B. Terms may be used more than once. Column A Column B ---------- 1. This radiation would be deflected towards a positively charged metal plate. A. atom ---------- 2. Atoms with the same number of protons but different numbers of neut ...
Glencoe Chapter 4 Structure of the Atom for the Wiki
... in the same ratio by mass. Law of Multiple Proportions Based on atomic theory but no experiment evidence at the time • The ratio of the masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. ...
... in the same ratio by mass. Law of Multiple Proportions Based on atomic theory but no experiment evidence at the time • The ratio of the masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. ...
- Physics
... electrical force. The neutrons do not repel each other. The energy released is not due to electrical energy. If momentum is conserved (it is) which objects have most of the kinetic energy, the large fission fragment nuclei or the neutrons? The objects released in nuclear fission carry on the order o ...
... electrical force. The neutrons do not repel each other. The energy released is not due to electrical energy. If momentum is conserved (it is) which objects have most of the kinetic energy, the large fission fragment nuclei or the neutrons? The objects released in nuclear fission carry on the order o ...
12_physics_notes_ch13_nuclei
... The number of protons in the nucleus is called the atomic number. It is denoted by Z. • Mass number: The total number of protons and neutrons present in a nucleus is called the mass number of the element. It is denoted by A. • No. of Protons, Electrons, nucleons and Neutrons in an Atom: a) Number of ...
... The number of protons in the nucleus is called the atomic number. It is denoted by Z. • Mass number: The total number of protons and neutrons present in a nucleus is called the mass number of the element. It is denoted by A. • No. of Protons, Electrons, nucleons and Neutrons in an Atom: a) Number of ...









![Review 2 key - Home [www.petoskeyschools.org]](http://s1.studyres.com/store/data/000860497_1-e3bea510ba504d09bc42d6f5e4936390-300x300.png)