* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Glencoe Chapter 4 Structure of the Atom for the Wiki
Periodic table wikipedia , lookup
Chemical bond wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Electric charge wikipedia , lookup
Electron configuration wikipedia , lookup
Chemical element wikipedia , lookup
Molecular dynamics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Matter wave wikipedia , lookup
Particle-size distribution wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Electron scattering wikipedia , lookup
Valley of stability wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of molecular theory wikipedia , lookup
History of chemistry wikipedia , lookup
Geiger–Marsden experiment wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Elementary particle wikipedia , lookup
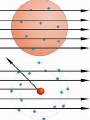
Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- “Uncut-Table” Indivisible parts which cannot be broken down further. Aristotle proposed that matter was continuous and was not made up of smaller particles. Fall of the Roman Empire; Dark Ages Medieval Period or the Middle Ages (4001400). Accepted until the 17th century (1600’s). Why? Renaissance Then came Isaac Newton and Robert Boyle. Robert Boyle : proved gas pressure and volume were related mathematically. John Dalton • logical hypothesis in 1800’s. Antoine Lavoisier French Chemist that discovered elemental oxygen Conservation of mass In ordinary chemical reactions, matter can be changed in many ways but it cannot be created or destroyed. Law of definite proportions • Joseph Proust specific substances always contain elements in the same ratio by mass. Law of Multiple Proportions Based on atomic theory but no experiment evidence at the time • The ratio of the masses of one element that combine with a constant mass of another element can be expressed in small whole numbers. Dalton’s Hypothesis What did Lavoisier and Proust finding explain? Basis for atomic theory. • All matter is composed of extreme small particles called atoms, which cannot be subdivided, created, or destroyed. • Atoms of a given element are identical in their physical and chemical properties. • Atoms of different elements differ in their physical and chemical properties. • Atoms of different elements combine in simple whole-number ratios to form compounds. • In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. Glencoe Chemistry: Chapter 4 Section Two William Crooke (1890’s) Used a cathode ray tube. An apparatus that helped discover the electron. • Electrode : metal piece in a cathode ray tube • Anode : positive electrode • Cathode : Negative electrode Cathode rays begin at the cathode and traveled toward the anode. J.J. Thomson (English) : • Researched with cathode rays and was credited with the discovery that cathode rays were actually electrons. • He was able to determine the ratio of an e- charge to its mass. Robert Millikan : • Used an oil drop experiment to obtain the first accurate measurements of an echarge. • Standard unit of negative charge (-1) symbol e- J. J. Thomson : • Found rays traveling in opposite direction of cathode rays. He showed these particles possessed a positive charge (+ charge). • Credited with discovery of proton (+ charge). It was a mass of 1836 times the mass of an e-. • 1920 ; Lord Rutherford (English) predicted a 3rd particle. • 1930 ; Walter Bothe - 1st evidence of this 3rd particle. • James Chadwick in 1932 found a high energy particle with no charge and the same mass of a proton; neutron. • The discovery of the neutron changed John Dalton’s atomic Theory. • The Gold Foil Experiment Page 112 Figure 4.12 & 4.13 • Subjecting a very thin sheet of Gold Foil to a stream of positively charged subatomic particles. • Found out that most of the particles passed through the sheet concluded that the atom was mostly empty space. Also found that a few particles were deflected. Rutherford explained the atom was composed of a small “core” containing all the positive charged and almost all of the mass, called the nucleus. The nucleus occupies about a trillionth of the volume of an atom (10-12). Hydrogen nucleus is the size of a ping-pong ball. The electron is roughly the size of a tennis ball, about 1.35 km away. • Atoms of the same element that differ in mass are called Isotopes. # Protons # Neutrons Neon-20 10 10 Neon-21 10 11 Neon-22 10 12 Henry Moseley (1913) Studied x-rays and found that the wavelength of the x-rays depend on the number of protons in the nucleus and always the same for a given element. Atomic number (Z) = number of protons. Number of protons determines the identity of the element. Nuclide a particular kind of atom containing a definite number of protons and neutrons. Mass number - Atomic number (Z) = number of Neutrons Isotopes of Hydrogen #Protons #Neutrons Mass# Protium Deuterium Tritium The Nuclear Atom Under Rutherford’s direction of Bohr, Geiger, and Marsden ; these scientists conducted experiments that are the basis for modern concept of atomic structure. Radioactivity Becquerel (1896) – Found that matter containing uranium exposes photographic film. Marie Curie and Pierre Curie found that rays were given off by the elements U and Ra Radioactivity is the phenomenon of “rays” beings produced spontaneously by unstable atomic nuclei. 1) Alpha particle: Helium nucleus consisted of 2p + 2n. 2) Beta particles – high speed electron released from a radioactive nuclei. β 3) Gamma Rays - high energy x-ray. γ Unstable nuclei that emit rays or particles to become stable are said to decay. Page 648 and Page 651.