Chem 1721 Brief Notes: Chapter 20 Chapter 20: Nuclear Chemistry
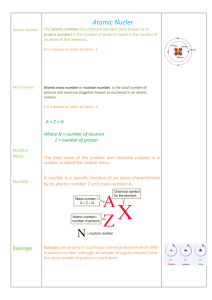
... atomic number = # protons in the nucleus; this does not change for atoms of a specific element i.e. every atom of calcium has 20 protons in the nucleus if the number of protons changes the identity of the element changes mass number = # protons + # neutrons; mass number can change because the number ...
... atomic number = # protons in the nucleus; this does not change for atoms of a specific element i.e. every atom of calcium has 20 protons in the nucleus if the number of protons changes the identity of the element changes mass number = # protons + # neutrons; mass number can change because the number ...
1 The Nucleus Total number of nucleons: mass number Number of
... Covers 200 isotopes, 35 elements Produces 2.4 neutron in average More neutrons are produced – may be explosive Size of the sample Too small: neutron escapes before striking a nucleus – subcritical Too large: neutrons are completely consumed – super critical In between: chain reactions keep at a co ...
... Covers 200 isotopes, 35 elements Produces 2.4 neutron in average More neutrons are produced – may be explosive Size of the sample Too small: neutron escapes before striking a nucleus – subcritical Too large: neutrons are completely consumed – super critical In between: chain reactions keep at a co ...
Chapter 25
... 1. What causes a transmutation of the nucleus to occur? 2. How are nuclear decay reaction equations balanced? 3. Do all radionuclides decay at the same rate? ...
... 1. What causes a transmutation of the nucleus to occur? 2. How are nuclear decay reaction equations balanced? 3. Do all radionuclides decay at the same rate? ...
Atomic Theory Handout CNS 8
... and the idea of "complementarity" -- that things may have a dual nature (as the electron is both particle and wave) but we can only experience one aspect at a time. ...
... and the idea of "complementarity" -- that things may have a dual nature (as the electron is both particle and wave) but we can only experience one aspect at a time. ...
Atomic Nuclei - RAJEEV Classes
... Two nuclides are isotones if they have the very same neutron number N, but different proton number Z. For example, boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones . ...
... Two nuclides are isotones if they have the very same neutron number N, but different proton number Z. For example, boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones . ...
notes ch 39 1st half Atomic Nucleus and Radioactivity
... the nucleus (positive charge attracts negative charge). • The neutrons do not have to equal the protons or electrons, and as such, they act a little like a nuclear cement holding the nucleus together. • The force holding protons and neutrons together is called the strong force. ...
... the nucleus (positive charge attracts negative charge). • The neutrons do not have to equal the protons or electrons, and as such, they act a little like a nuclear cement holding the nucleus together. • The force holding protons and neutrons together is called the strong force. ...
Article 2: Key Concepts and Vocabulary
... including the conditions under which they are stable or unstable. Our Sun is a plasma whose core is at a temperature of 15.6 million degrees Celsius. The temperatures in fusion research reactors are even higher. ...
... including the conditions under which they are stable or unstable. Our Sun is a plasma whose core is at a temperature of 15.6 million degrees Celsius. The temperatures in fusion research reactors are even higher. ...
Content Domain III: Chemistry—Atomic Theory and
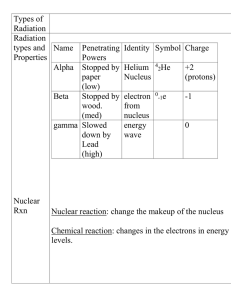
... After decaying, radioactive atoms change into other atoms. During alpha decay, the nucleus loses two protons and two neutrons. During beta decay, the nucleus gains a proton and loses a neutron. During gamma decay, the energy content of the nucleus changes but the atomic number of the element does no ...
... After decaying, radioactive atoms change into other atoms. During alpha decay, the nucleus loses two protons and two neutrons. During beta decay, the nucleus gains a proton and loses a neutron. During gamma decay, the energy content of the nucleus changes but the atomic number of the element does no ...
Atomic and Nuclear Physics
... • Nucleus of Uranium-235 splits by collision with a neutron to produce 2 daughter nuclei and a small number of neutrons (3) • This process releases energy in the form of kinetic energy (= thermal energy) of the 2 nuclei (fission products) • The neutrons produced by one fission can strike other U-235 ...
... • Nucleus of Uranium-235 splits by collision with a neutron to produce 2 daughter nuclei and a small number of neutrons (3) • This process releases energy in the form of kinetic energy (= thermal energy) of the 2 nuclei (fission products) • The neutrons produced by one fission can strike other U-235 ...
The Band of Stability
... Radioactive decay changes the nature of an atom’s nucleus, and it happens for a reason. Each element from hydrogen (atomic number 1) to lead (atomic number 82) has stable isotopes in which the tendency of protons to repel one another is overcome by attractive nuclear forces. These attractive nuclear ...
... Radioactive decay changes the nature of an atom’s nucleus, and it happens for a reason. Each element from hydrogen (atomic number 1) to lead (atomic number 82) has stable isotopes in which the tendency of protons to repel one another is overcome by attractive nuclear forces. These attractive nuclear ...
Nuclear Fission
... • Radioactive atoms: unstable atoms that decay and emit particles and energy from their nuclei – Not all elements are radioactive • Most cases it is only certain isotopes that are radioactive – Example: »H – 1 = »H – 2 = »H – 3 = ...
... • Radioactive atoms: unstable atoms that decay and emit particles and energy from their nuclei – Not all elements are radioactive • Most cases it is only certain isotopes that are radioactive – Example: »H – 1 = »H – 2 = »H – 3 = ...
Biology Fall Semester Test 1 Study Guide
... Two products of cellular respiration are: In producers, chlorophyll and sunlight are necessary for the process of: The closing of its shell when a clam is removed from its watery environment is an example of how a clam maintains its: In a trophic pyramid, _______% of the energy from a source is pass ...
... Two products of cellular respiration are: In producers, chlorophyll and sunlight are necessary for the process of: The closing of its shell when a clam is removed from its watery environment is an example of how a clam maintains its: In a trophic pyramid, _______% of the energy from a source is pass ...
Unit 3 – Atomic Structure
... • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into smaller nuclei • Nuclear fusion - Combining nuclei of light elements into a ...
... • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into smaller nuclei • Nuclear fusion - Combining nuclei of light elements into a ...
atoms - Groupfusion.net
... Normally, this is the same for every atom of an element, therefore number of protons identifies the element. In a “normal”, neutral atom, number of protons = number of electrons Mass Number = the number of protons + number of neutrons = A Number of neutrons in an atom of an element can vary. These a ...
... Normally, this is the same for every atom of an element, therefore number of protons identifies the element. In a “normal”, neutral atom, number of protons = number of electrons Mass Number = the number of protons + number of neutrons = A Number of neutrons in an atom of an element can vary. These a ...
Chapter 16 – Nuclear Energy
... • Uranium fuel rods, control rods, water used to cool and control chain reactions, vessel that surrounds the fuel rods. ...
... • Uranium fuel rods, control rods, water used to cool and control chain reactions, vessel that surrounds the fuel rods. ...