* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atom and Nucleus. Radioactivity. Nuclear Energy.
Muon-catalyzed fusion wikipedia , lookup
Technetium-99m wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear drip line wikipedia , lookup
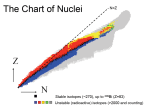
Lecture 13 Atom and Nucleus. Radioactivity. Nuclear Energy. • • • • Rutherford Model of the Atom Radioactivity Nuclear Energy Binding Energy Rutherford Model of the Atom J.J. Thomson discovered electron in 1897. He suggested an atomic model in which electrons were embedded in spread positive charge. Ernest Rutherford attempted to test this model by bombarding a thin gold foil with alpha-particles. A significant scattering of the alpha-particles was detected. Rutherford suggested a new model of the atom which resembled a mini solar system. Nuclear Structure The nucleus contains protons and neutrons (discovered in 1932). They both are referred to as nucleons. Atoms of different chemical elements have different number of protons (atomic number) Atoms with the same number of protons and different number of neutrons are called isotopes A nucleus with a particular composition is called a nuclide. 1 H – hydrogen atom with 1 proton in the nucleus 1 Radioactivity Radioactivity was discovered accidentally in 1896. A.Becquerel found that uranium (U) exposed photographic film. In other words, uranium emitted penetrating radiation. Marie and Pierre Curie soon discovered 2 more radioactive elements, which were called polonium and radium. Radioactivity is associated with the nucleus and is not affected by chemical reactions or heating. Radioactive Decay It was found that magnetic field splits the radiation from radioactive materials into 3 parts. Alpha ()particles positively charged Beta () particles negatively charged electrons Gamma () particles (rays) no charge The nucleus composition does not change in gamma decay, but changes in alpha- and beta-decay. -decay occurs in large nuclei, which are not stable. The heaviest stable isotope is 20983Bi. -decay transforms a neutron into a proton (n p+ + e) Electron capture: p+ + e n and p+ n + e+ Half-Life The half-life of a radionuclide is the period of time needed for half of any initial amount of the nuclide to decay. 1 mg 22688Ra 0.5 mg 22286Rn for 1600 years Radionuclides have unchanging half-life. This is the base for archaeological dating. Half-life for 14C is 5730 years. Radiation from radionuclides carries high energy. It is harmful for living tissues. 1 sievert (Sv) 1 J of radiation from X-rays or -rays absorbed by 1 kg of body tissue. A permissible limit is ~ 20mSv a year. Nuclear Energy Our everyday life units for energy and mass are not suitable for atoms. The atomic mass unit: 1u = 1.66 1027 kg Mass of a hydrogen atom is 1.0078 u The energy unit is the electronvolt. This is the energy an electron gains being accelerated by a potential difference of 1 V. 1eV = 1.60 1019 J 1Mev = 1.60 1013 J E (1 u) = mc2 = 931 MeV Binding Energy Hydrogen has 3 isotopes: protium, deuterium, and tritium. The mass of 11H is 1.0078 u , the mass of a neutron is 1.0087 u . The sum is 2.0165 u . However, the mass of a deuterium atom is 2.0141 u , 0.0024 u = 2.2 MeV less than the combined mass of p+n. All atoms have smaller masses than the sum of masses of their nucleons. Some mass and energy is given off due to action of forces that hold the protons and neutrons together. This energy is called binding energy. Binding Energy Binding energy of a deuterium atom is 2.2 MeV (1.1 MeV per nucleon) , of a 20983Bi is 1640 MeV (7.8 MeV per nucleon). A typical binding energy is ~800 billion kJ/kg To boil water it takes 2260 kJ/kg The most stable element is iron (5626Fe). Fission and Fusion If a heavy nucleus is split into 2 medium-size ones, each of the new nuclei will have more binding energy per nucleon. Joining 2 light nuclei would result in a nucleus with more binding energy per nucleon. The extra energy will be given off. Splitting a large nucleus is called nuclear fission. Joining light nuclei is called nuclear fusion. Summary Atomic nuclei consist of protons and neutrons. Large nuclei are unstable and radioactively decay. Radiation released by this process is dangerous for living creatures. The binding energy curve allows for two types of nuclear reactions: fusion of light elements and fission of heavy elements.