Nuclear Decay
... Fission is a reaction when the nucleus of an atom, having captured a neutron, splits into two or more nuclei, and in so doing, releases a significant amount of energy as well as more neutrons. These neutrons then go on to split more nuclei and a chain reaction takes place. ...
... Fission is a reaction when the nucleus of an atom, having captured a neutron, splits into two or more nuclei, and in so doing, releases a significant amount of energy as well as more neutrons. These neutrons then go on to split more nuclei and a chain reaction takes place. ...
Nuclear Chemistry powerpoint
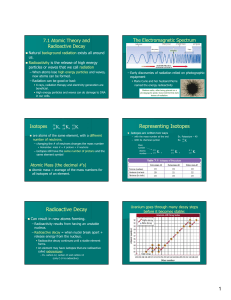
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Principles of Technology
... In order to study nuclear reactions and their products, scientists have invented a number of detection devices. The principle underlying all these devices is that the particles produced in nuclear reactions leave their “fingerprints” as they pass through the detector. In a Geiger counter, an electri ...
... In order to study nuclear reactions and their products, scientists have invented a number of detection devices. The principle underlying all these devices is that the particles produced in nuclear reactions leave their “fingerprints” as they pass through the detector. In a Geiger counter, an electri ...
Nuclear Chemistry powerpoint
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
... ) and no charge ( ). Thus, it causes change in or numbers. Gamma rays almost accompany alpha and beta radiation. However, since there is effect on mass number or atomic number, they are usually from nuclear equations. ...
Nuclear Decay
... Alpha particles, beta particles and gamma rays all pose a danger to living tissue because they can ionize, or strip the electrons from, atoms. Types of radiation that can ionize atoms are known as ionization radiation. When this makes contact with living tissue, it can result in burns, tumours and o ...
... Alpha particles, beta particles and gamma rays all pose a danger to living tissue because they can ionize, or strip the electrons from, atoms. Types of radiation that can ionize atoms are known as ionization radiation. When this makes contact with living tissue, it can result in burns, tumours and o ...
The Nature of Molecules
... nucleus of an atom; one level contains only 1 orbit of electrons, others contain 4 different orbits of electrons (each orbit is filled with 2 e-’s) • The filling of orbitals and energy levels relates to the chemical behavior of atoms • The number of electrons of an atom relates to its ...
... nucleus of an atom; one level contains only 1 orbit of electrons, others contain 4 different orbits of electrons (each orbit is filled with 2 e-’s) • The filling of orbitals and energy levels relates to the chemical behavior of atoms • The number of electrons of an atom relates to its ...
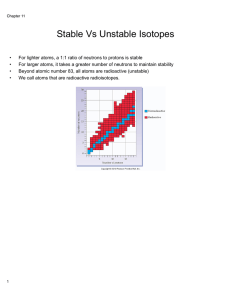
Stable Vs Unstable Isotopes
... The emission of a particle from an unstable nucleus is called fission. nuclear decay. mutation. translocation. fusion. Radioactivity is generally associated with which part of the atom? the entire atom electrons protons nucleus neutrons Which of the properties of radioisotopes make them useful as tr ...
... The emission of a particle from an unstable nucleus is called fission. nuclear decay. mutation. translocation. fusion. Radioactivity is generally associated with which part of the atom? the entire atom electrons protons nucleus neutrons Which of the properties of radioisotopes make them useful as tr ...
Atoms and Spectral Lines
... • Atoms: Made up of three subatomic particles – proton: Mass - 1.7x10-24 gm. It takes 600,000,000,000,000,000,000,000 protons to make a gram! Each carries one positive electric charge. – neutron: Very similar to proton, but no charge. – electron: Mass 1/2000th of the proton. Carries one negative cha ...
... • Atoms: Made up of three subatomic particles – proton: Mass - 1.7x10-24 gm. It takes 600,000,000,000,000,000,000,000 protons to make a gram! Each carries one positive electric charge. – neutron: Very similar to proton, but no charge. – electron: Mass 1/2000th of the proton. Carries one negative cha ...
Test Review: Unit 1 - Ms. Hill`s Pre
... i. q= energy expressed in the units of joules or calories ii. m= mass express in the units of g or kg iii. ΔT = change in temperature (temperature final – temperature initial) expressed in the units of Celsius or Kelvin. Remember ΔT oC = ΔT K 27. Can you solve for energy required (+q) or lost (-q)? ...
... i. q= energy expressed in the units of joules or calories ii. m= mass express in the units of g or kg iii. ΔT = change in temperature (temperature final – temperature initial) expressed in the units of Celsius or Kelvin. Remember ΔT oC = ΔT K 27. Can you solve for energy required (+q) or lost (-q)? ...
No Slide Title
... are randomly distributed and cancel out. For the ones in z, we get a net magnetization proportional to Na - Nb. • Since this is (more or less) the situation in a real sample, we will from now on use Mo in all further descriptions/examples. • There is an important difference between a m and Mo. While ...
... are randomly distributed and cancel out. For the ones in z, we get a net magnetization proportional to Na - Nb. • Since this is (more or less) the situation in a real sample, we will from now on use Mo in all further descriptions/examples. • There is an important difference between a m and Mo. While ...
Chemistry Vocab for Quiz 12/21 or 12/22 Atom – The smallest
... Atomic mass – The average mass of one atom of an element Proton – A small positively particle in the nucleus Neutron – a small particle in the nucleus with no charge Electron – A tiny negatively charge particle that moves around the nucleus of the atom Nucleus –The central core of the atom containin ...
... Atomic mass – The average mass of one atom of an element Proton – A small positively particle in the nucleus Neutron – a small particle in the nucleus with no charge Electron – A tiny negatively charge particle that moves around the nucleus of the atom Nucleus –The central core of the atom containin ...
Inside the Atom connections to the lower secondary (KS3
... • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemistry modules. Students are introduced to a simple atomic model and chemical reactions as the rearrangements of atoms. Using this as a starting poin ...
... • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemistry modules. Students are introduced to a simple atomic model and chemical reactions as the rearrangements of atoms. Using this as a starting poin ...
Nuclear Chemistry
... • Small nuclei combine to form heavier, more stable nuclei. • No radioactive products are produced. • Fusion reactions are easier to control, so the risk of explosions & accidents are decreased. ...
... • Small nuclei combine to form heavier, more stable nuclei. • No radioactive products are produced. • Fusion reactions are easier to control, so the risk of explosions & accidents are decreased. ...
Chapter 21 Nuclear Chemistry - Ocean County Vocational
... • Small nuclei combine to form heavier, more stable nuclei. • No radioactive products are produced. • Fusion reactions are easier to control, so the risk of explosions & accidents are decreased. ...
... • Small nuclei combine to form heavier, more stable nuclei. • No radioactive products are produced. • Fusion reactions are easier to control, so the risk of explosions & accidents are decreased. ...
Nuclear Chemistry
... greater than 56 the nucleus should fall apart to become more stable. Fusion 98% of all matter in the Universe is made of hydrogen and helium At its conception only the lightest element, hydrogen was around but later as the universe expanded, stars were born when the hydrogen clouds collapsed under g ...
... greater than 56 the nucleus should fall apart to become more stable. Fusion 98% of all matter in the Universe is made of hydrogen and helium At its conception only the lightest element, hydrogen was around but later as the universe expanded, stars were born when the hydrogen clouds collapsed under g ...