Effects of atomic electrons on nuclear stability and radioactive decay
... A straightforward analysis of the database [6] shows that all stable isotopes without an exception correspond to minima of atomic masses MA(Z) on the isobars. Moreover, all -decay and K-capture processes that are energetically allowed are realized in nature (no other prohibitions are involved). Thu ...
... A straightforward analysis of the database [6] shows that all stable isotopes without an exception correspond to minima of atomic masses MA(Z) on the isobars. Moreover, all -decay and K-capture processes that are energetically allowed are realized in nature (no other prohibitions are involved). Thu ...
Notes on Atomic Structure atoms
... same proportions (by mass and by number) of its elements This means a given compound always has the same composition, regardless of where it came from. ...
... same proportions (by mass and by number) of its elements This means a given compound always has the same composition, regardless of where it came from. ...
Shiny, Happy Pretest - Alex LeMay – Science
... ___25. There is a positively charged nucleus in large mostly empty space; the electrons are somewhere in that space. ___26. The atom was one solid ball. ___27. Electrons orbit in probability clouds ___28. The positively charged electron ball is studded with electrons, like blueberries in a muffin. ...
... ___25. There is a positively charged nucleus in large mostly empty space; the electrons are somewhere in that space. ___26. The atom was one solid ball. ___27. Electrons orbit in probability clouds ___28. The positively charged electron ball is studded with electrons, like blueberries in a muffin. ...
PYP001-122-Final Exam Solution [Choice A is the correct
... B) Like charges attract one another, but opposite charges repel each other. C) Neutrons and electrons attract each other. D) Electrons are found inside the nucleus of an atom. E) Neutrons and protons repel each other. Q22. Which of the following materials is a good insulator? A) All of these. B) Gla ...
... B) Like charges attract one another, but opposite charges repel each other. C) Neutrons and electrons attract each other. D) Electrons are found inside the nucleus of an atom. E) Neutrons and protons repel each other. Q22. Which of the following materials is a good insulator? A) All of these. B) Gla ...
Unit 16 Worksheet - Jensen Chemistry
... Name_________________________________________________period____________Unit 16: reaction rates Review: 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus ...
... Name_________________________________________________period____________Unit 16: reaction rates Review: 1. When do electrons release photons(packets of energy)? When the electrons: a. move to higher levels of energy b. return to their original energy level c increase orbital speed around the nucleus ...
Dalton`s Atomic Theory Discovery of Electron Properties of Cathode
... Postulates of Bohr’s Atomic Model The main postulates of Bohr’s Model are given below: 1. Electrons revolve around the nucleus in a fixed orbit. 2. As long as electron revolves in a fixed orbit it does not emit and absorb energy. Hence energy of electron remains constant. 3. The orbit nearest to the ...
... Postulates of Bohr’s Atomic Model The main postulates of Bohr’s Model are given below: 1. Electrons revolve around the nucleus in a fixed orbit. 2. As long as electron revolves in a fixed orbit it does not emit and absorb energy. Hence energy of electron remains constant. 3. The orbit nearest to the ...
Chap 3 Atomic Structure
... Dalton’s Atomic Theory The important postulates of Dalton’s atomic theory are: 1. All elements are composed of atoms. Atom is too small so that it could not be divided into further simpler components. 2. Atom cannot be destroyed or produced. 3. Atoms of an element are similar in all respects. They h ...
... Dalton’s Atomic Theory The important postulates of Dalton’s atomic theory are: 1. All elements are composed of atoms. Atom is too small so that it could not be divided into further simpler components. 2. Atom cannot be destroyed or produced. 3. Atoms of an element are similar in all respects. They h ...
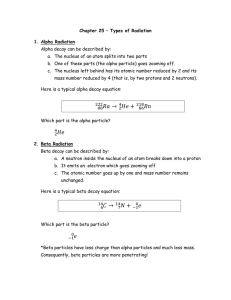
Chapter 25 – Types of Radiation 1. Alpha Radiation Alpha decay
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
Chemistry Review - pams-hoey
... nucleus 2. Radioactive decay: The spontaneous breakdown of an unstable atomic nucleus 3. Decay Series: The series of steps by which a radioactive nucleus decays into a non-radioactive nucleus. 4. Alpha Decay: Occurs when a nucleus releases an alpha particle 5. Beta Decay: Loses a beta particle causi ...
... nucleus 2. Radioactive decay: The spontaneous breakdown of an unstable atomic nucleus 3. Decay Series: The series of steps by which a radioactive nucleus decays into a non-radioactive nucleus. 4. Alpha Decay: Occurs when a nucleus releases an alpha particle 5. Beta Decay: Loses a beta particle causi ...
The Nature of Matter
... • Ex: gold (Au) has an atomic #of 79 and an atomic mass of 197. How many neutrons are in gold? – 197-79=118 neutrons ...
... • Ex: gold (Au) has an atomic #of 79 and an atomic mass of 197. How many neutrons are in gold? – 197-79=118 neutrons ...
Lesson 13: Nuclear Propulsion Basics
... – Bond together by the strong nuclear force • Stronger than the electrostatic force binding electrons to the nucleus or repelling protons from one another • Limited in range to a few x 10-15 m ...
... – Bond together by the strong nuclear force • Stronger than the electrostatic force binding electrons to the nucleus or repelling protons from one another • Limited in range to a few x 10-15 m ...
Nuclear Magnetic Resonance Spectroscopy
... they generate a magnetic field. Nuclei which have an odd number of protons can behave as if they were tiny bar magnets. Of importance to organic chemists is that the hydrogen nucleus, one proton, and the carbon-13 nucleus, 13 protons, both have this property. We will discuss proton magnetic resonanc ...
... they generate a magnetic field. Nuclei which have an odd number of protons can behave as if they were tiny bar magnets. Of importance to organic chemists is that the hydrogen nucleus, one proton, and the carbon-13 nucleus, 13 protons, both have this property. We will discuss proton magnetic resonanc ...
Nuclear Fission and Fusion
... the heart of long dead stars. Along with nearly every other element that we have on earth. ...
... the heart of long dead stars. Along with nearly every other element that we have on earth. ...
Adobe Acrobat file () - Wayne State University Physics and
... have the same kinetic energy. (a). Conservation of momentum requires the momenta of the two fragments be equal in magnitude and oppositely directed. Thus, from KE = p2/2m, the lighter alpha particle has more kinetic energy that the more massive daughter nucleus. ...
... have the same kinetic energy. (a). Conservation of momentum requires the momenta of the two fragments be equal in magnitude and oppositely directed. Thus, from KE = p2/2m, the lighter alpha particle has more kinetic energy that the more massive daughter nucleus. ...
Atomic Structure, the Periodic Table, and Nuclear Radiation
... – The closer an electron is to the nucleus, the more strongly it is attracted. – The more protons in a nucleus, the more strongly an electron is attracted. 2. Electrons are repelled by other electrons in an atom. So, if other electrons are between a valence electron and the nucleus, the valence elec ...
... – The closer an electron is to the nucleus, the more strongly it is attracted. – The more protons in a nucleus, the more strongly an electron is attracted. 2. Electrons are repelled by other electrons in an atom. So, if other electrons are between a valence electron and the nucleus, the valence elec ...