7.2- Nuclear reactions (PPT)
... Nuclear fusion ▪ For a more massive star, there is enough gravity to fuse the elements all the way up to iron. ▪ But there can be no more fusion when the star is completely iron. Why? ▪ Since the radiation pressure now ceases, gravity is no longer balanced and the star collapses into a neutron star ...
... Nuclear fusion ▪ For a more massive star, there is enough gravity to fuse the elements all the way up to iron. ▪ But there can be no more fusion when the star is completely iron. Why? ▪ Since the radiation pressure now ceases, gravity is no longer balanced and the star collapses into a neutron star ...
sch3u unit 1 test: matter
... 9. When fluorine forms an ionic bond it tends to a. lose electrons b. gain electrons c. share electrons d. lose protons 10. Copper (II) hydroxide is composed of a. 2 elements, 2 atoms b. 2 elements, 3 atoms c. 3 elements, 4 atoms d. 3 elements, 5 atoms ...
... 9. When fluorine forms an ionic bond it tends to a. lose electrons b. gain electrons c. share electrons d. lose protons 10. Copper (II) hydroxide is composed of a. 2 elements, 2 atoms b. 2 elements, 3 atoms c. 3 elements, 4 atoms d. 3 elements, 5 atoms ...
Chemical Change
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
particle - Uplift North Hills
... Neutrinos are elementary particles that travel close to the speed of light, lack an electric charge, are able to pass through ordinary matter almost undisturbed and are thus extremely difficult to detect It took 26 years before the neutrino was actually detected. As of 1999, it is believed neutrinos ...
... Neutrinos are elementary particles that travel close to the speed of light, lack an electric charge, are able to pass through ordinary matter almost undisturbed and are thus extremely difficult to detect It took 26 years before the neutrino was actually detected. As of 1999, it is believed neutrinos ...
File
... • nuclei fuse together • for very light elements, stability increases with increasing mass ...
... • nuclei fuse together • for very light elements, stability increases with increasing mass ...
Atomic Structure Notes
... Dalton’s Atomic Theory (1808) 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. 3. The atoms of one element are different from the atoms of all other elements. 4. Atoms of one elemen ...
... Dalton’s Atomic Theory (1808) 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. 3. The atoms of one element are different from the atoms of all other elements. 4. Atoms of one elemen ...
Nuclear and Radiation Section - University of Toronto Physics
... necessary to postulate another force of nature - the so-called strong force – that comes into play only at a distance of less than 10-15 m or so. This very short-range strong force does not distinguish between neutrons and protons, being attractive between any two nucleons (p-p, p-n, n-n); it is the ...
... necessary to postulate another force of nature - the so-called strong force – that comes into play only at a distance of less than 10-15 m or so. This very short-range strong force does not distinguish between neutrons and protons, being attractive between any two nucleons (p-p, p-n, n-n); it is the ...
-30- Section 9: f"
... c. Which quantum number (n, l, ml or ms) describes the quantization of the direction of an electron's orbital angular momentum? d. When the electrons in a substance bind into Cooper pairs, what does the substance become? e. Classical physics and the equipartition theorem predict the specific heat of ...
... c. Which quantum number (n, l, ml or ms) describes the quantization of the direction of an electron's orbital angular momentum? d. When the electrons in a substance bind into Cooper pairs, what does the substance become? e. Classical physics and the equipartition theorem predict the specific heat of ...
Radioactivity2015
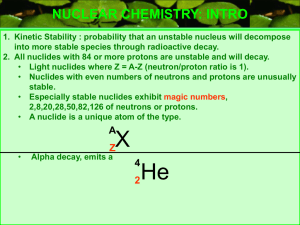
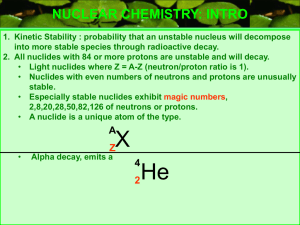
... • This particle, which consists of two protons and two neutrons, has a net positive charge. • Although emitted with high energy, alpha particles lose energy quickly as they pass through matter of air and therefore, do not travel long distances. • They can even be stopped by a piece of paper or the o ...
... • This particle, which consists of two protons and two neutrons, has a net positive charge. • Although emitted with high energy, alpha particles lose energy quickly as they pass through matter of air and therefore, do not travel long distances. • They can even be stopped by a piece of paper or the o ...
File - Mr. Walsh`s AP Chemistry
... named by describing the molecular formula, using prefixes for the numbers. o You will need to memorize the number prefixes for the numbers 1–10. o E.g., P2O5 is diphosphorus pentoxide. **Note that the prefix “mono—“ is never used with the first element. SO3 is simply sulfur trioxide. However, “mono— ...
... named by describing the molecular formula, using prefixes for the numbers. o You will need to memorize the number prefixes for the numbers 1–10. o E.g., P2O5 is diphosphorus pentoxide. **Note that the prefix “mono—“ is never used with the first element. SO3 is simply sulfur trioxide. However, “mono— ...
Notes Atoms
... made of matter has mass Most of the mass of an atom is found in the nucleus • About 99.9% of the mass of an atom is in the nucleus Nuclear Particles The two particles in the nucleus, protons and neutrons, make up 99.9 % of the mass of the atom ...
... made of matter has mass Most of the mass of an atom is found in the nucleus • About 99.9% of the mass of an atom is in the nucleus Nuclear Particles The two particles in the nucleus, protons and neutrons, make up 99.9 % of the mass of the atom ...