Chapter 21
... Life on Earth evolved along three major lines, called domains, all derived from a common ancestor. Each domain contains several phyla. The domains, Bacteria and Archaea, remained prokaryotic, whereas the third, Eukarya, evolved into the modern eukaryotic cell. ...
... Life on Earth evolved along three major lines, called domains, all derived from a common ancestor. Each domain contains several phyla. The domains, Bacteria and Archaea, remained prokaryotic, whereas the third, Eukarya, evolved into the modern eukaryotic cell. ...
N x C (N-2)
... There is certainly no lack of small membrane-bound vesicles in the eukaryotic cell! But these vesicles can be divided into basically two types: those that are fully derived from the RER/golgi system and those that are not. The latter are the so-called microbodies, of variable size but often smaller ...
... There is certainly no lack of small membrane-bound vesicles in the eukaryotic cell! But these vesicles can be divided into basically two types: those that are fully derived from the RER/golgi system and those that are not. The latter are the so-called microbodies, of variable size but often smaller ...
Kreb`s Cycle - robertschem
... 14. Why is FAD used instead of NAD+? At one step of Krebs cycle, succinate is oxidized to become fumarate with the help of FAD. The energy involved succinate-fumarate reaction does not allow NAD+ to be reduced sufficiently. FAD is lower-energy and is able to help oxidize succinate in the process (an ...
... 14. Why is FAD used instead of NAD+? At one step of Krebs cycle, succinate is oxidized to become fumarate with the help of FAD. The energy involved succinate-fumarate reaction does not allow NAD+ to be reduced sufficiently. FAD is lower-energy and is able to help oxidize succinate in the process (an ...
Option B Rev A
... •4 ATP generated from SLP •2 NADH + H+ enter electron transport chain to produce ATP ...
... •4 ATP generated from SLP •2 NADH + H+ enter electron transport chain to produce ATP ...
CHAPTER 6
... the forearm muscle of a human subjected to 19 minutes of exercise. Note that the three P atoms of ATP (a ,b, and g) have different chemical shifts, reflecting their different chemical ...
... the forearm muscle of a human subjected to 19 minutes of exercise. Note that the three P atoms of ATP (a ,b, and g) have different chemical shifts, reflecting their different chemical ...
Electron attachment to molecular clusters by collisional charge transfer
... of n strongly dependent on experimental conditions. The other amendment is use of a seeded supersonic expansion to produce the fast alkali atom beam; this provides a sufficiently high atom flux to offset the relative weakness of the cluster beam. This method for electron attachment to molecular clus ...
... of n strongly dependent on experimental conditions. The other amendment is use of a seeded supersonic expansion to produce the fast alkali atom beam; this provides a sufficiently high atom flux to offset the relative weakness of the cluster beam. This method for electron attachment to molecular clus ...
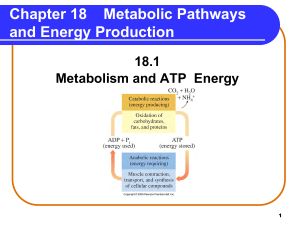
Metabolic Pathways and Energy Production
... groups for the production of ATP? In reaction 7, phosphate groups from two 1,3bisphosphoglycerate molecules are transferred to ADP to form two ATP. In reaction 10, phosphate groups from two phosphoenolpyruvate molecules are used to form two more ATP. ...
... groups for the production of ATP? In reaction 7, phosphate groups from two 1,3bisphosphoglycerate molecules are transferred to ADP to form two ATP. In reaction 10, phosphate groups from two phosphoenolpyruvate molecules are used to form two more ATP. ...
Bacterial Classification
... Glucose + Pi Glucose-6-PO4 + H2O ΔG = +13.8 kJ/mol, Keq = 5 x 10-3 ATP + H20 ADP + Pi ΔG = -30.5 kJ/mol, Keq = 4 x 105 Glucose + ATP Glucose-6-PO4 + ADP ΔG = (-30.5 kJ/mol) + (+13.8 kJ/mol) = -16.7 kJ/mol ...
... Glucose + Pi Glucose-6-PO4 + H2O ΔG = +13.8 kJ/mol, Keq = 5 x 10-3 ATP + H20 ADP + Pi ΔG = -30.5 kJ/mol, Keq = 4 x 105 Glucose + ATP Glucose-6-PO4 + ADP ΔG = (-30.5 kJ/mol) + (+13.8 kJ/mol) = -16.7 kJ/mol ...
File
... C6H12O6 + 6CO2 == 36 ATP + 6CO2 + 6H2O is the same as saying Glucose + Oxygen == Carbon Dioxide + Water + Energy (ATP). Oxygen (O2) is consumed as glucose (C6H12O6) is broken down into carbon dioxide (CO2) and water (H2O). The energy released in the form of ATP is captured by the cell and used to do ...
... C6H12O6 + 6CO2 == 36 ATP + 6CO2 + 6H2O is the same as saying Glucose + Oxygen == Carbon Dioxide + Water + Energy (ATP). Oxygen (O2) is consumed as glucose (C6H12O6) is broken down into carbon dioxide (CO2) and water (H2O). The energy released in the form of ATP is captured by the cell and used to do ...
overview, inorgs, trace nutrients
... • Incorporated into electron carriers (free and proteinbound). • Deficiency leads to cheilosis. ...
... • Incorporated into electron carriers (free and proteinbound). • Deficiency leads to cheilosis. ...
Slide 1
... From one molecule of glucose, glycolysis yields 2 NADH, the link reaction yields 2 NADH and the Krebs cycle yields 6 NADH and 2 FADH2. 10 × 2.5 = 25 ATP from NADH ...
... From one molecule of glucose, glycolysis yields 2 NADH, the link reaction yields 2 NADH and the Krebs cycle yields 6 NADH and 2 FADH2. 10 × 2.5 = 25 ATP from NADH ...
Enduring Understanding: Growth, reproduction and maintenance of
... Essential Knowledge 2.A.2: Organisms capture and store free energy for use in biological processes ...
... Essential Knowledge 2.A.2: Organisms capture and store free energy for use in biological processes ...
Atoms and bonds in molecules and chemical
... of the event from a set of true propositions involving at least a scientific law or principle. The unification approach intends to derive the occurrence of the event using a theory that unifies many phenomena or the theory that unifies the phenomena better than any other. In the causal model the exp ...
... of the event from a set of true propositions involving at least a scientific law or principle. The unification approach intends to derive the occurrence of the event using a theory that unifies many phenomena or the theory that unifies the phenomena better than any other. In the causal model the exp ...
Metabolism Part II: The tricarboxylic acid (TCA), citric acid, or Krebs
... A fourth NAD+ coenzyme was reduced to NADH when PVru\,ateu,asoxidizedto&etyl CuA in thestep bridyinRgly&lysis and the'rCA cycle.'l'hrtv more ATP are produced when this coenzyme is recycled, and a total of 15 ATP are therefore synthesized for each pyruvate ion oxidized in the mitochondria of the cell ...
... A fourth NAD+ coenzyme was reduced to NADH when PVru\,ateu,asoxidizedto&etyl CuA in thestep bridyinRgly&lysis and the'rCA cycle.'l'hrtv more ATP are produced when this coenzyme is recycled, and a total of 15 ATP are therefore synthesized for each pyruvate ion oxidized in the mitochondria of the cell ...
Calvin Cycle
... RuBP Carboxylase causes a conformational change to a "closed" conformation in which access of solvent water to the active site is blocked. ...
... RuBP Carboxylase causes a conformational change to a "closed" conformation in which access of solvent water to the active site is blocked. ...
SEPARATION OF MITOCHONDRIAL MEMBRANES OF
... the release of latent activity which is not assayable when compartmentation is intact, and in the elimination of permeability or other factors which might normally limit the amount of pyruvate or thiamine pyrophosphate (TPP) cofactor accessible to the AAS . It is not surprising that the levels of va ...
... the release of latent activity which is not assayable when compartmentation is intact, and in the elimination of permeability or other factors which might normally limit the amount of pyruvate or thiamine pyrophosphate (TPP) cofactor accessible to the AAS . It is not surprising that the levels of va ...
Lecture 13: Krebs` Cycle / Citric Acid
... chain (cytochromes) are only electron carriers i.e. they cannot give or take protons (H+) During the electron transport, FAD and the iron atom of different cytochromes get successively reduced (Fe++) and oxidized (Fe+++) and enough energy is released in some places which is utilized in the photophos ...
... chain (cytochromes) are only electron carriers i.e. they cannot give or take protons (H+) During the electron transport, FAD and the iron atom of different cytochromes get successively reduced (Fe++) and oxidized (Fe+++) and enough energy is released in some places which is utilized in the photophos ...
citric acid cycle
... The urea cycle and the reactions that feed amino group into it. Note that the enzymes catalyzing these reactions are distributed between the mitochondrial matrix and the cytosol. One amino group enters the urea cycle from carbamoyl phosphate (step 1), formed in the matrix; the other (entering at s ...
... The urea cycle and the reactions that feed amino group into it. Note that the enzymes catalyzing these reactions are distributed between the mitochondrial matrix and the cytosol. One amino group enters the urea cycle from carbamoyl phosphate (step 1), formed in the matrix; the other (entering at s ...
Bio301 final exam 2005 with model answers
... wastewater. Explain what the difficulties are to have both processes running in parallel and how SND can be accomplished. Nitrification involves the aerobic oxidation of ammonia, the key inorganic nitrogen compound in waste water to nitrate or nitrite. It is catalysed by strictly aerobic bacteria th ...
... wastewater. Explain what the difficulties are to have both processes running in parallel and how SND can be accomplished. Nitrification involves the aerobic oxidation of ammonia, the key inorganic nitrogen compound in waste water to nitrate or nitrite. It is catalysed by strictly aerobic bacteria th ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.