Chapter 9
... Hans Adolf Krebs – received the Nobel Prize in 1953 for his work on the series of chemical reactions known as the tricarboxylic acid cycle (also called the citric acid cycle, or Krebs cycle). This is the basic system for the essential pathway of oxidation within the cell. Copyright © 2005 Pearson E ...
... Hans Adolf Krebs – received the Nobel Prize in 1953 for his work on the series of chemical reactions known as the tricarboxylic acid cycle (also called the citric acid cycle, or Krebs cycle). This is the basic system for the essential pathway of oxidation within the cell. Copyright © 2005 Pearson E ...
SOLUTE TRANSPORT
... Sugar Phosphotransferase System of Gram-Negative Bacteria is classic example: ...
... Sugar Phosphotransferase System of Gram-Negative Bacteria is classic example: ...
Bio-Energetics - mynoteslibrary
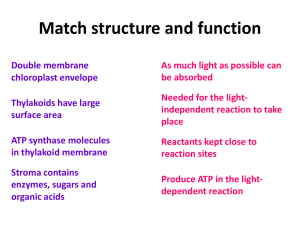
... yellow and orange. These are present in the thylakoid membrane along with two kinds of chlorophyll. Carotenoids can absorb wavelength of light that chlorophyll can not absorb and transfer to chlorophyll-a. On the other side excessive light can damage chlorophyll. Instead of transmitting energy to ch ...
... yellow and orange. These are present in the thylakoid membrane along with two kinds of chlorophyll. Carotenoids can absorb wavelength of light that chlorophyll can not absorb and transfer to chlorophyll-a. On the other side excessive light can damage chlorophyll. Instead of transmitting energy to ch ...
Which of the following molecules is most likely to be used in a
... is that one gram of fat contains more molecules of fat than a gram of carbohydrates because the molecules fit together more closely and because the carbohydrates are hydrophilic and may include some water in their overall structure. In addition, in considering a carbohydrate and a fat of similar mol ...
... is that one gram of fat contains more molecules of fat than a gram of carbohydrates because the molecules fit together more closely and because the carbohydrates are hydrophilic and may include some water in their overall structure. In addition, in considering a carbohydrate and a fat of similar mol ...
Cellular Respiration - McGraw Hill Higher Education
... cell produce energy if oxygen is limited? Fermentation is an anaerobic process that produces a limited amount of ATP in the absence of oxygen. In animal cells, including human cells, pyruvate, the end product of glycolysis, is reduced by NADH to lactate (Fig. 8.5). Depending on their particular enzy ...
... cell produce energy if oxygen is limited? Fermentation is an anaerobic process that produces a limited amount of ATP in the absence of oxygen. In animal cells, including human cells, pyruvate, the end product of glycolysis, is reduced by NADH to lactate (Fig. 8.5). Depending on their particular enzy ...
Fatty Acid Catabolism
... 1. Which lipid form is transported across the inner mitochondrial membrane before β-oxidation? A) Acylcarnitine. B) Fatty acyl CoA. C) Acetoacetyl CoA. D) Lysophospholipid CoA. 2. There are four steps in the β-oxidation pathway. Some reaction types are listed below. Give the proper reaction types i ...
... 1. Which lipid form is transported across the inner mitochondrial membrane before β-oxidation? A) Acylcarnitine. B) Fatty acyl CoA. C) Acetoacetyl CoA. D) Lysophospholipid CoA. 2. There are four steps in the β-oxidation pathway. Some reaction types are listed below. Give the proper reaction types i ...
respiration revision quiz
... a. Glycolysis: Glucose (….C) is broken down to produce 2 molecules of Pyruvate (….C). b. The link reaction: Pyruvate is dehydrogenated (………………… is removed) and decarboxylated (…………………. is removed) and converted ...
... a. Glycolysis: Glucose (….C) is broken down to produce 2 molecules of Pyruvate (….C). b. The link reaction: Pyruvate is dehydrogenated (………………… is removed) and decarboxylated (…………………. is removed) and converted ...
Oxidation of Carbohydrate
... – High net ATP yield but slow ATP production – Must be broken down into free fatty acids (FFAs) and glycerol – Only FFAs are used to make ATP ...
... – High net ATP yield but slow ATP production – Must be broken down into free fatty acids (FFAs) and glycerol – Only FFAs are used to make ATP ...
video slide - Green River Community College
... • In cellular respiration, glucose and other organic molecules are broken down in a series of steps • Electrons from organic compounds are usually first transferred to NAD+, a coenzyme • As an electron acceptor, NAD+ functions as an oxidizing agent during cellular respiration • Each NADH (the reduce ...
... • In cellular respiration, glucose and other organic molecules are broken down in a series of steps • Electrons from organic compounds are usually first transferred to NAD+, a coenzyme • As an electron acceptor, NAD+ functions as an oxidizing agent during cellular respiration • Each NADH (the reduce ...
Popeye knew what he was doing!
... Photosynthesis & Cellular Respiration Fermentation • Occurs without oxygen, and is seen in some types of bacteria, yeast, and fatigued muscle cells. • Fermentation occurs only in the cytoplasm (no ETC involved). After glycolysis, 1 or 2 reactions occur to reduce pyruvate to another compound. • Ferme ...
... Photosynthesis & Cellular Respiration Fermentation • Occurs without oxygen, and is seen in some types of bacteria, yeast, and fatigued muscle cells. • Fermentation occurs only in the cytoplasm (no ETC involved). After glycolysis, 1 or 2 reactions occur to reduce pyruvate to another compound. • Ferme ...
Slide 1
... All of these processes represent a significant amount of electron transfer to oxygen without concomitant ATP synthesis and all become much more active when exercising hard, creating interesting complications when trying to interpret oxygen consumption and its association with athletic performance. W ...
... All of these processes represent a significant amount of electron transfer to oxygen without concomitant ATP synthesis and all become much more active when exercising hard, creating interesting complications when trying to interpret oxygen consumption and its association with athletic performance. W ...
Ch2
... • Energy substrate for prolonged, less intense exercise – High net ATP yield but slow ATP production – Must be broken down into free fatty acids (FFAs) and glycerol – Only FFAs are used to make ATP ...
... • Energy substrate for prolonged, less intense exercise – High net ATP yield but slow ATP production – Must be broken down into free fatty acids (FFAs) and glycerol – Only FFAs are used to make ATP ...
Overview of ATP Production
... carbohydrates, they must transfer the energy to molecules of ATP. – ATP is composed of adenine, ribose, and three phosphate groups. – ATP transfers energy to many different chemical reactions; almost all metabolic pathways directly or indirectly run on energy supplied by ATP. ATP Production - Dion ...
... carbohydrates, they must transfer the energy to molecules of ATP. – ATP is composed of adenine, ribose, and three phosphate groups. – ATP transfers energy to many different chemical reactions; almost all metabolic pathways directly or indirectly run on energy supplied by ATP. ATP Production - Dion ...
NADH - Mrs. Yu`s Science Classes
... - 2 NADH are produced - 4 ATP are produced - 2 pyruvate are formed. In summary, glycolysis takes 1 glucose and turns it into 2 pyruvate, 2 NADH, and a net of 2 ATP (made 4 ATP, but used 2 ATP). ...
... - 2 NADH are produced - 4 ATP are produced - 2 pyruvate are formed. In summary, glycolysis takes 1 glucose and turns it into 2 pyruvate, 2 NADH, and a net of 2 ATP (made 4 ATP, but used 2 ATP). ...
LOCATION: CYTOPLASM
... Inhibited by G-6-P which accumulated if other reactions are inhibited. 2. Pyruvate kinase 4 isoenzymic forms inhibited by ATP, activated by F-1,6-Bis P see fig 12.17 Horton liver form also inhibited by phosphorylation 3. PHOSPHOFRUCTOKINASE Main point of regulation ATP, citrate AMP, F-2,6-bisP i ...
... Inhibited by G-6-P which accumulated if other reactions are inhibited. 2. Pyruvate kinase 4 isoenzymic forms inhibited by ATP, activated by F-1,6-Bis P see fig 12.17 Horton liver form also inhibited by phosphorylation 3. PHOSPHOFRUCTOKINASE Main point of regulation ATP, citrate AMP, F-2,6-bisP i ...
THE CITRIC ACID CYCLE
... • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to ...
... • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to ...
Restricted rotation of the amino group of nucleic acid base
... shifts of 8H and 2H protons are slightly dependent on the concentration of BrU. The tendency of concentration dependence of 8H proton has a good correlation with the behavior of the lower field amino proton signal and that of 2H proton corresponds to the higher field amino proton signal. Taking into ...
... shifts of 8H and 2H protons are slightly dependent on the concentration of BrU. The tendency of concentration dependence of 8H proton has a good correlation with the behavior of the lower field amino proton signal and that of 2H proton corresponds to the higher field amino proton signal. Taking into ...
Preparation for Exam 1
... pathways in cells for providing energy. You also were shown anabolic pathways: gluconeogenesis, glycogen synthesis, pentose phosphate. Glycogenolysis (glycogen breakdown) fell in the cracks between glycolysis and gluconeogenesis. Study these pathways by structure and know the intermediates that lead ...
... pathways in cells for providing energy. You also were shown anabolic pathways: gluconeogenesis, glycogen synthesis, pentose phosphate. Glycogenolysis (glycogen breakdown) fell in the cracks between glycolysis and gluconeogenesis. Study these pathways by structure and know the intermediates that lead ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.